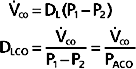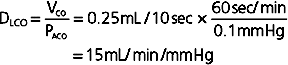CHAPTER 23 Oxygen and Carbon Dioxide Transport
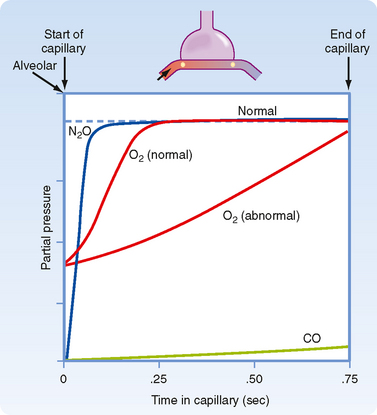
The respiratory and circulatory systems function together to transport sufficient oxygen (O2) from the lungs to the tissues to sustain normal cellular activity and to transport carbon dioxide (CO2) from the tissues to the lungs for expiration. CO2, a product of active cellular glucose metabolism, is transported from the tissues via systemic veins to the lungs, where it is expired (Fig. 23-1). To enhance uptake and transport of these gases between the lungs and tissues, specialized mechanisms (e.g., O2-hemoglobin binding and HCO3− transport of CO2) have evolved that enable O2 uptake and CO2 expiration to occur simultaneously. Moreover, these specialized mechanisms facilitate uptake of O2 and expiration of CO2. To gain an understanding of the mechanisms involved in the transport of these gases, one must consider gas diffusion properties, as well as transport and delivery mechanisms.
GAS DIFFUSION
Gases in the Lung Diffuse from Regions of Higher to Lower Partial Pressure
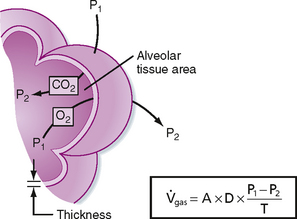
The process of gas diffusion is passive and similar whether diffusion occurs in a gaseous or liquid state. The rate of diffusion of a gas through a liquid is described by Graham’s law, which states that the rate is directly proportional to the solubility coefficient of the gas and inversely proportional to the square root of its molecular weight. Calculation of the diffusion properties for O2 and CO2 reveals that CO2 diffuses approximately 20 times faster than O2 does. Rates of O2 diffusion from the lungs into blood and from blood into tissue, and vice versa for CO2, are predicted by Fick’s law of gas diffusion (Fig. 23-2). Fick’s law states that the diffusion of a gas ( gas) across a sheet of tissue is directly related to the surface area (A) of the tissue, the diffusion constant (D) of the specific gas, and the partial pressure difference (P1 − P2) of the gas on each side of the tissue and is inversely related to tissue thickness (T). That is,
gas) across a sheet of tissue is directly related to the surface area (A) of the tissue, the diffusion constant (D) of the specific gas, and the partial pressure difference (P1 − P2) of the gas on each side of the tissue and is inversely related to tissue thickness (T). That is,
Oxygen and Carbon Dioxide Exchange in the Lung Is Perfusion Limited
The high affinity of CO for Hgb enables large amounts of CO to be taken up in blood with little or no appreciable increase in its partial pressure. Gases that are chemically bound to Hgb do not exert a partial pressure in blood. Like CO, both CO2 and O2 have relatively low solubility in the alveolar-capillary membrane but high solubility in blood because of their ability to bind to Hgb. However, their rate of equilibration is sufficiently rapid for complete equilibration to occur during the transit time of the red blood cell within the capillary. Equilibration for O2 and CO2 usually occurs within 0.25 second. Thus, O2 and CO2 transfer is normally perfusion limited. The partial pressure of a diffusion limited gas (i.e., CO) does not reach equilibrium with the alveolar pressure over the time that it spends in the capillary (Fig. 23-3). Although CO2 has a greater rate of diffusion in blood than O2 does, it has a lower membrane-blood solubility ratio and consequently takes approximately the same amount of time to reach equilibration in blood.
OXYGEN TRANSPORT
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


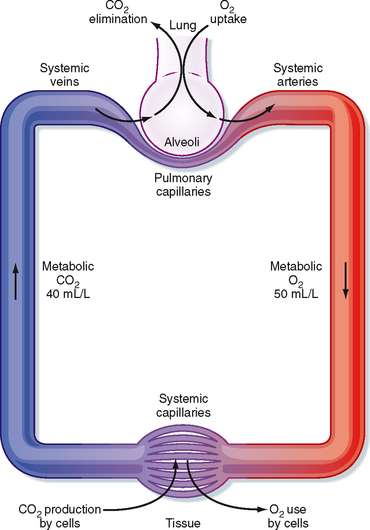
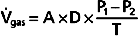
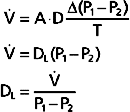
 and the average partial pressure of CO in the alveolus. That is,
and the average partial pressure of CO in the alveolus. That is,