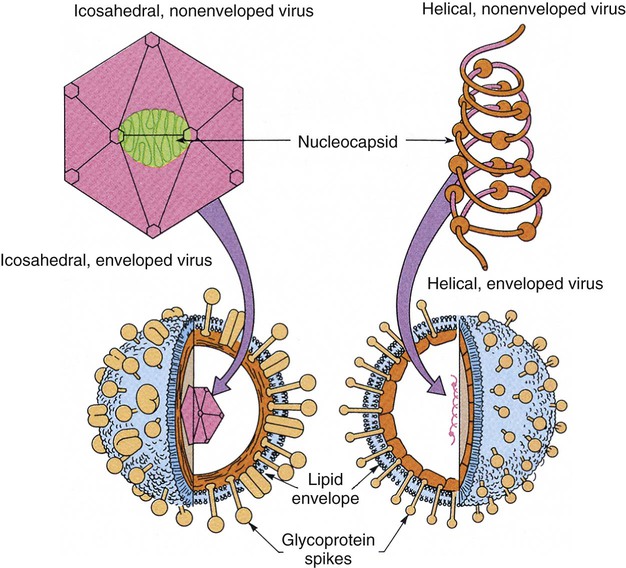
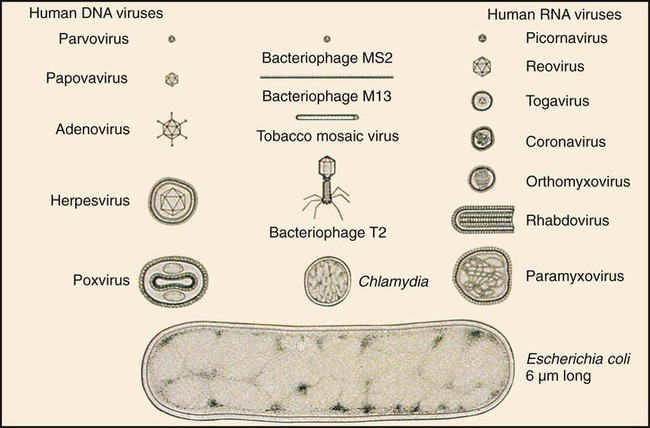
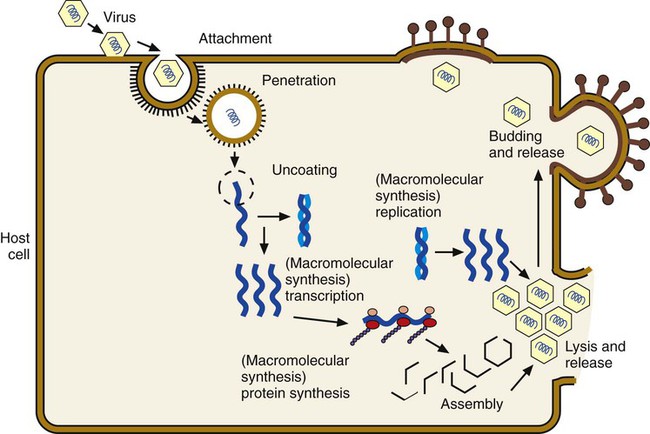
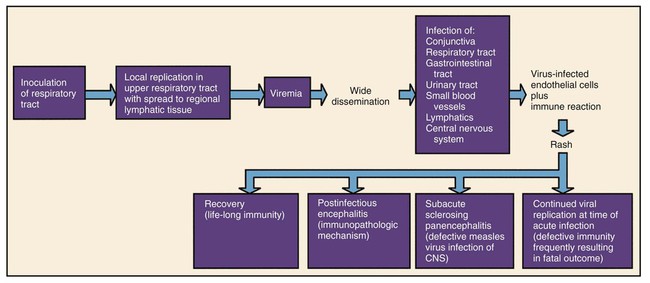
Chapter 65 1. Describe the physical components that make up a virion and list a function for each component. 2. Define the viral infectious cycle, including naming the six steps in this process. 3. Explain viral tropism and provide a specific example. 4. Define the properties used to classify a virus and identify the person responsible for classification of a virus. 5. Explain the steps in viral pathogenesis. 6. List some of the reasons the clinical science industry has seen an increased demand for clinical viral services. 7. Name some of the equipment necessary to set up a clinical virology laboratory and give the function of each piece. 8. List some of the viruses associated with the following clinical specimens: throat or nasopharyngeal swab or aspirate, urine, stool, lesion, blood, bone marrow, and stool or rectal swab. 9. List some of the most efficient laboratory tests for detecting the following viruses: enterovirus, herpes simplex virus, influenza virus, norovirus, and respiratory syncytial virus (RSV). 10. Define the Tzanck test and list the viruses for which the test is used. 11. Define monolayer, primary cells, semicontinuous (low passage) cells, and continuous cells. 12. Explain the types of cell lines used in viral cell culture; describe their similarities and differences. 13. Define and differentiate cell culture growth medium and maintenance medium. 14. Explain the incubation conditions for routine cell cultures. 15. Define CPE and explain how it is rated when reading cell cultures. 16. Describe a shell vial cell culture and explain its advantages over conventional cell culture. 17. Define the hemadsorption procedure and name the viruses for which the test is used. 18. Name the virus family capable of establishing viral latency in the human dorsal nerve root ganglion and explain the possible consequence of the latency. 19. Name the preferred tissue type of cell culture for growth of the following viruses: influenza A virus, varicella-zoster virus, herpes virus, and cytomegalovirus (CMV). 20. Associate an appropriate viral pathogen with the following viral syndromes: infant croup, infant bronchiolitis, adult gastroenteritis, parotitis, infectious mononucleosis, and meningitis. The emergence of a new viral disease across a very large geographical region (worldwide) with prolonged human-to-human transmission is called a pandemic. To date, most of the pandemics recorded have been caused by an influenza virus. Pandemics result when an influenza virus undergoes a genetic shift and the reassortment of genes combines with those of another organism, usually an animal. The resulting virus emerges as a completely new or “novel” virus. The genetic changes in viral genomes may result from antigenic shift (major changes that result in novel viral antigens) and/or antigenic drift (minor changes that occur infrequently), which are discussed in Chapter 66. One of the most deadly influenza outbreaks was the Spanish Flu pandemic of 1918-1919. This pandemic was associated with infection with a novel influenza virus of avian origin. After a period of adaptation in humans, the virus emerged in pandemic form and was responsible for more than 50 million deaths worldwide, including 500,000 in the United States. What was so different about this pandemic was that it affected young and healthy individuals, not just the very young or very old. The more recent influenza pandemic of the twentieth century was associated with a human influenza virus in which genes reassorted in combination with an avian influenza virus. Virus particles, referred to as virions, consist of two or three parts: • An inner nucleic acid core, consisting of either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) • A protein coat that surrounds and protects the nucleic acid (the capsid) • In some of the larger viruses, a lipid-containing envelope that surrounds the virus Because enveloped viruses are very susceptible to drying out and destruction in the environment, they typically are transmitted by direct contact, such as respiratory, sexual, or parenteral contact. This prevents exposure to the environment and successful propagation of the viral agent to another susceptible host. Viruses that do not have an envelope are often referred to as “naked” viruses. Naked viruses are very resistant to environmental factors. Because of their stability, they typically are transmitted by the fecal-oral route. Many viruses have glycoprotein spikes extending from their surface. The term nucleocapsid is often used to describe the nucleic acid genome surrounded by a symmetric protein coat (Figure 65-1). The viral capsid protects the viral genome and is responsible for the tropism to specific cell types in naked viruses. Viral capsids typically are composed of repeating structural subunits referred to as capsomeres. The capsomeres associate to form the capsid and a characteristic symmetric structure. The most common capsid structures geometrically form a helical or icosahedral structure (see Figure 65-1). Icosahedral capsids are cubical and have 20 flat sides; irregularly shaped capsids usually assume a helical form and are spiral shaped. As mentioned, in some viruses the nucleocapsid is enclosed in a lipid envelope. The envelope is responsible for viral entry into the host cell (see Figure 65-1). During the infectious process, enveloped virions bud from a host cell’s cytoplasmic, nuclear, or endoplasmic reticular membrane, and a portion of the membrane remains attached to the virion as the viral envelope. Inserted into this viral envelope are viral proteins, such as hemagglutinin (HA), neuraminidase, or glycoprotein spikes. The glycoprotein spikes assist in stabilization of attachment for the lipid envelope and for attachment to the host cell to facilitate viral entry. Some enveloped viruses also contain a matrix protein that lies between the envelope and the nucleocapsid. The matrix protein may have enzymatic activities and/or biologic functions related to infection, such as inhibition of host-cell transcription. Viruses that cause disease in humans range from approximately 20 to 300 nm. Even the largest viruses, such as the poxviruses, cannot be detected with a light microscope, because they are less than one fourth the size of a staphylococcal cell (Figure 65-2). Not until the invention of the electron microscope in the 1930s were viruses visualized. The electron microscope’s improved magnification (more than 100,000 times) allowed visualization of virus particles and paved the way for viral classification based on structural components. Viruses are strict intracellular parasites, reproducing or replicating only inside a host cell. The six steps of virus replication, called the infectious cycle, proceed as follows (Figure 65-3). 1. Attachment, also referred to as adsorption, is the first step of the infectious cycle. It involves recognition of a suitable host cell and specific binding between viral capsid proteins (often the glycoprotein spikes) and the carbohydrate receptor of the host cell. Each type of virus specifically recognizes and attaches to a specific type of host cell, allowing infection of some tissues but not others (viral tropism, as previously described). 2. Penetration is the process by which viruses enter the host cell. One mechanism of penetration involves fusion of the viral envelope with the host cell membrane. This method not only provides a mechanism for internalizing the virus, but also leads to fusion between the infected host cell and additional nearby host cells, forming multinucleated cells called syncytia. Detection of syncytia can be used to determine the presence of virus in cell cultures or stained smears of clinical specimens. Other mechanisms of viral penetration include phagocytosis by host cells (endocytosis) or injection of viral nucleic acid. 3. Uncoating occurs once the virus has been internalized. It is the process by which the capsid is removed; this may be by degradation of viral enzymes or host enzymes or by simple dissociation. Uncoating is necessary to release the viral genome before the viral DNA or RNA is delivered to its intracellular site of replication in the nucleus or cytoplasm. 4. Macromolecular synthesis involves the production of nucleic acid and protein polymers. Viral transcription leads to the synthesis of messenger RNA (mRNA), which encodes early and late viral proteins. Early proteins are nonstructural elements, such as enzymes, and late proteins are structural components. Rapid identification of virus in a cell culture can be accomplished by detecting early viral proteins in infected cells using immunofluorescent staining techniques. Replication of viral nucleic acid is necessary to provide genomes for progeny virus particles or virions. Macromolecular synthesis varies, depending on the organization of the viral genome. 5. Viral assembly is the process by which structural proteins, genomes, and in some cases viral enzymes are assembled into virus particles. Envelopes are acquired during viral “budding” from a host cell membrane. Nuclear endoplasmic reticulum and cytoplasmic membranes are common areas for budding. Acquisition of an envelope is the final step in viral assembly. 6. Release of intact virus particles occurs after cell lysis (lytic virus) or by budding from cytoplasmic membranes. Release by budding may not result in rapid host cell death, as does release by cell lysis. Detection of virus in cell cultures is facilitated by recognition of areas of cell lysis. Detection of virus released by budding is more difficult, because the cell monolayer remains intact. Influenza viruses, which are released by budding with minimal cell destruction, can be detected in cell culture by an alternative technique called hemadsorption. Influenza virus–infected cells contain virally encoded glycoprotein hemagglutinins inserted into the host cell’s cytoplasmic membrane, preparing for inclusion in the viral envelope at the time of release by cytoplasmic budding. Red blood cells (RBCs) added to the culture medium adsorb to the outer membranes of infected cells but not to uninfected cells. Each infected host cell results in as many as 100,000 virions; however, as few as 1% of these may be infectious or “viable” in the practical sense. Noninfectious viral particles may result from errors or mutations that occur during the infectious cycle. Examples of the variety of pathogenic mechanisms of viral infection are illustrated in disease caused by infection with the measles virus. After replication in the upper respiratory tract and subsequent viremia, the virus infects many susceptible cells throughout the body, including endothelial cells in capillaries of the skin. This is accompanied by local inflammation and results in the characteristic rash of measles. Immunocompetent individuals eradicate the virus, resolving the infection, and have lifelong immunity. In some, antibody produced in response to the measles infection cross reacts with tissue in the central nervous system (CNS), causing a postinfectious encephalitis. In others, slow but continuing replication of damaged virus in the brain gives rise to subacute sclerosing panencephalitis. In severely immunocompromised individuals, ongoing primary infection is not aborted by the usual immune mechanisms, and the result is death (Figure 65-4). Because the measles virus is not an oncogenic virus, no cancers result from prolonged infection. An increased understanding of viral structure and replication has improved the availability of therapeutic agents for the treatment of viral infections. Approximately 40 antiviral drugs are formally licensed for clinical use, and about half of these are used in the treatment of human immunodeficiency virus (HIV) infections. Antivirals also are used in the treatment of herpes viruses (herpes simplex virus [HSV], varicella-zoster virus [VZV], and cytomegalovirus [CMV]), hepatitis B virus (HBV), hepatitis C virus (HCV), respiratory syncytial virus (RSV), and the influenza viruses. (Antiviral agents are reviewed in more detail in Chapter 67.) Hundreds of viruses cause disease in humans. Viruses of human medical importance comprise four orders, 25 families, 13 subfamilies and 66 genera. Individual viruses may cause multiple different diseases, and conversely, many viruses may cause the same disease, all of which complicates the understanding of viral disease in humans. For example, viruses that can cause encephalitis include HSV, many arboviruses, rabies virus, HIV, and measles virus. However, HSV also can cause pharyngitis, genital infection, conjunctivitis, and encephalitis. Viruses that are important pathogens in humans and the viral syndromes they cause are summarized in Table 65-1. Specific viral agents and their role in human disease are discussed in Chapter 66. TABLE 65-1 Viral Syndromes and Common Viral Pathogens CMV, cytomegalovirus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; HTLV-1, human T-lymphotropic virus type 1; JCV, JC virus; SARS, severe acute respiratory syndrome; VZV, varicella-zoster virus. When determining which virology tests to offer, each clinical laboratory should decide whether the test is required for the appropriate care of their patient population and whether techniques are available that provide an accurate, cost-effective test result. Viral diseases that require laboratory diagnosis include sexually transmitted diseases, diarrhea, respiratory disease in adults and children, aseptic meningitis, arbovirus encephalitides, congenital diseases, hepatitis, and infections in immunocompromised individuals. Table 65-2 presents a representative sample of viruses identified in a community clinical virology laboratory. TABLE 65-2 Viruses Detected by Culture, PCR, or Assay for Antigen in a Community Hospital Virology Laboratory* PCR, Polymerase chain reaction. *Data from 1 year of testing at Evanston Northwestern Healthcare, Evanston, Illinois. Laboratory scientists in a clinical virology laboratory must be familiar with cell culture, enzyme immunoassay, immunofluorescence methods, and molecular methods (e.g., PCR), in addition to other common laboratory techniques. Large equipment needed for a full-service virology laboratory includes a laminar flow biologic safety cabinet (BSC), fluorescence microscope, inverted bright field microscope, refrigerated centrifuge, incubator, refrigerator and freezer, roller drum for holding cell culture tubes during incubation, and enzyme or molecular testing instrumentation (Figures 65-5 to 65-7). Appropriate specimen selection dictates that the specimen type and suspected viruses should be included on the requisition. The laboratory should always be notified if rare agents representing a danger to laboratory workers are suspected (e.g., SARS coronavirus, H5N1 avian influenza virus, hemorrhagic fever viruses). Serum for serologic testing may be necessary, and some viral diseases should be considered during specific months. Table 65-3 presents specimens for the diagnosis of viral diseases, noting trends in seasonality. TABLE 65-3 Specimens for Diagnosis of Viral Diseases* CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; EIA, enzyme immunoassay; EM, electron microscopy; F, fall; PCR, polymerase chain reaction; HIV, human immunodeficiency virus; S, summer; SARS, severe acute respiratory syndrome; SP, spring; W, winter; Y, Year-round. *Specimens indicated beside specific viruses should be obtained if that specific virus is suspected (++++, most appropriate; + least appropriate). †Direct fluorescent antibody studies are available for herpes simplex virus and varicella-zoster virus. Viral specimens should be processed in a BSC whenever possible (see Figure 65-7). This protects specimens from contamination by the processing technologist and protects those in the laboratory from infectious aerosols created when specimens are manipulated. Latex gloves and a laboratory coat should be worn during manipulation of all patient specimens. Vortexing, pipetting, and centrifugation can create dangerous aerosols. Vortexing should be done in a tightly capped tube behind a shield. After vortexing, the tube should be opened in a BSC. Pipetting should be performed behind a protective shield. Pipettes must be discarded into a disinfectant fluid so that the disinfectant reaches the inside of the pipette or into a leak-proof biosafety bag for autoclaving or incineration. When patient cell cultures are manipulated, such as during inoculation or feeding (exchange of cell culture medium), only one patient sample or series of cell culture tubes should be open at one time. Aerosols and microsplashes contribute to cross-contamination of cultures, especially during viral respiratory season when a high percentage of specimens are positive for influenza virus, RSV, and other viruses. Processing virology specimens is not complicated (Table 65-4). In general, any primary specimen or swab specimen that may be contaminated with bacteria or fungi should be added to a viral transport medium. Normally sterile fluid specimens can be inoculated directly to cell culture. The viral transport medium or fluid specimens not in a transport medium should be vortexed immediately before inoculation to break up virus-containing cells and resuspend the inoculum. Adding sterile glass beads to the transport medium helps break up cell clumps and release virus from cell aggregates. This may not be necessary, because some commercially available mediums contain beads. Grossly contaminated or potentially toxic specimens, such as minced or ground tissue, can be centrifuged (1000× g for 15 minutes) and the virus-containing supernatant used as the inoculum. Each viral cell culture tube is inoculated with 200 to 400 µL of specimen. If insufficient specimen is available, the specimen obtained is diluted with a viral transport medium to increase the volume. Excess specimen can be stored at –70°C in the event the initial culture is contaminated. A set of uninoculated cultures should be maintained simultaneously for continual monitoring of sterility and contamination throughout the process. TABLE 65-4 Laboratory Processing of Viral Specimens
Overview of the Methods and Strategies in Virology*
General Characteristics
Viral Structure
Viral Replication
Pathogenesis and Spectrum of Disease
Prevention and Therapy
Antiviral Agents
Viruses That Cause Human Diseases
Viral Syndrome
Viral Pathogens
Infants and Children
Upper respiratory tract infection
Rhinovirus, coronavirus, parainfluenza, adenovirus, respiratory syncytial virus, influenza
Pharyngitis
Adenovirus, coxsackie A, herpes simplex virus, Epstein-Barr virus, rhinovirus, parainfluenza, influenza
Croup
Parainfluenza, respiratory syncytial virus, metapneumovirus
Bronchitis
Parainfluenza, respiratory syncytial virus, metapneumovirus
Bronchiolitis
Respiratory syncytial virus, parainfluenza, metapneumovirus
Pneumonia
Respiratory syncytial virus, adenovirus, influenza, parainfluenza
Gastroenteritis
Rotavirus, adenovirus 40-41, calicivirus, astrovirus
Congenital and neonatal disease
HSV-2, echovirus, and other enteroviruses, CMV, parvovirus B19, VZV, HIV, hepatitis viruses
Adults
Upper respiratory tract infection
Rhinovirus, coronavirus, adenovirus, influenza, parainfluenza, Epstein-Barr virus
Pneumonia
Influenza, adenovirus, sin nombre virus (hantavirus), severe acute respiratory syndrome (SARS) coronavirus
Pleurodynia
Coxsackie B
Gastroenteritis
Noroviruses
Cervical cancer
Human papillomavirus
All Patients
Parotitis
Mumps, parainfluenza
Myocarditis/pericarditis
Coxsackie B and echoviruses
Keratitis/conjunctivitis
Herpes simplex virus, VZV, adenovirus, enterovirus 70
Pleurodynia
Coxsackie B
Herpangina
Coxsackie A
Febrile illness with rash
Echoviruses and coxsackie viruses
Infectious mononucleosis
Epstein-Barr virus and CMV
Meningitis
Echoviruses and coxsackie viruses, mumps, lymphocytic choriomeningitis, HSV-2
Encephalitis
HSV-1, togaviruses, bunyaviruses, flaviviruses, rabies, enteroviruses, measles, HIV, JCV
Hepatitis
Hepatitis A, B, C, D (delta agent), E, and non-A, B, C, D, E
Hemorrhagic cystitis
Adenovirus, BK virus
Cutaneous infection with or without rash
HSV-1 and HSV-2; VZV; enteroviruses; measles; rubella; parvovirus B-19; human herpes virus 6 and 7; HPV; poxviruses, including smallpox, monkeypox, molluscum contagiosum; and orf
Hemorrhagic fever
Ebola, Marburg, Lassa, yellow fever, dengue, and other viruses
Generalized, no specific target organ
HIV-1, HIV-2, HTLV-1
Laboratory Diagnosis
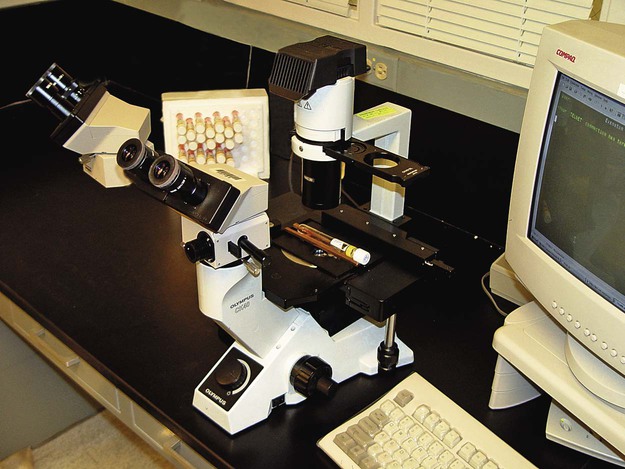
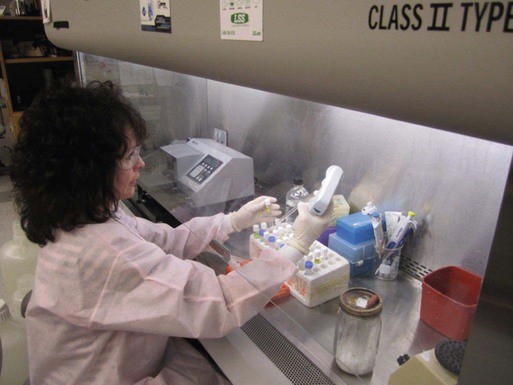
Setting Up a Clinical Virology Laboratory
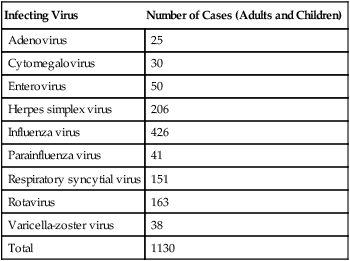
Infecting Virus
Number of Cases (Adults and Children)
Adenovirus
25
Cytomegalovirus
30
Enterovirus
50
Herpes simplex virus
206
Influenza virus
426
Parainfluenza virus
41
Respiratory syncytial virus
151
Rotavirus
163
Varicella-zoster virus
38
Total
1130
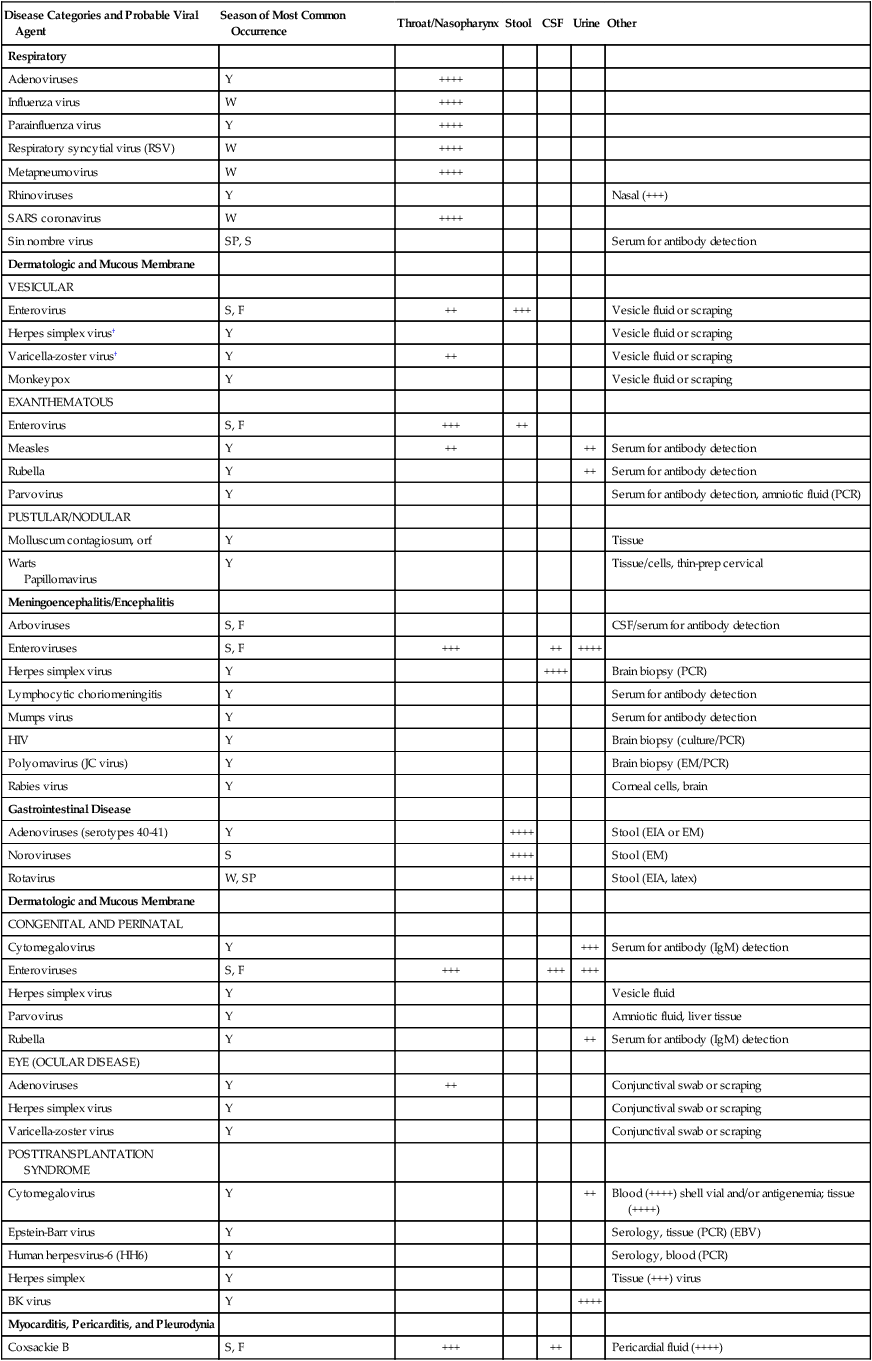
Specimen Selection and Collection
General Principles
Disease Categories and Probable Viral Agent
Season of Most Common Occurrence
Throat/Nasopharynx
Stool
CSF
Urine
Other
Respiratory
Adenoviruses
Y
++++
Influenza virus
W
++++
Parainfluenza virus
Y
++++
Respiratory syncytial virus (RSV)
W
++++
Metapneumovirus
W
++++
Rhinoviruses
Y
Nasal (+++)
SARS coronavirus
W
++++
Sin nombre virus
SP, S
Serum for antibody detection
Dermatologic and Mucous Membrane
VESICULAR
Enterovirus
S, F
++
+++
Vesicle fluid or scraping
Herpes simplex virus†
Y
Vesicle fluid or scraping
Varicella-zoster virus†
Y
++
Vesicle fluid or scraping
Monkeypox
Y
Vesicle fluid or scraping
EXANTHEMATOUS
Enterovirus
S, F
+++
++
Measles
Y
++
++
Serum for antibody detection
Rubella
Y
++
Serum for antibody detection
Parvovirus
Y
Serum for antibody detection, amniotic fluid (PCR)
PUSTULAR/NODULAR
Molluscum contagiosum, orf
Y
Tissue
Warts
Papillomavirus
Y
Tissue/cells, thin-prep cervical
Meningoencephalitis/Encephalitis
Arboviruses
S, F
CSF/serum for antibody detection
Enteroviruses
S, F
+++
++
++++
Herpes simplex virus
Y
++++
Brain biopsy (PCR)
Lymphocytic choriomeningitis
Y
Serum for antibody detection
Mumps virus
Y
Serum for antibody detection
HIV
Y
Brain biopsy (culture/PCR)
Polyomavirus (JC virus)
Y
Brain biopsy (EM/PCR)
Rabies virus
Y
Corneal cells, brain
Gastrointestinal Disease
Adenoviruses (serotypes 40-41)
Y
++++
Stool (EIA or EM)
Noroviruses
S
++++
Stool (EM)
Rotavirus
W, SP
++++
Stool (EIA, latex)
Dermatologic and Mucous Membrane
CONGENITAL AND PERINATAL
Cytomegalovirus
Y
+++
Serum for antibody (IgM) detection
Enteroviruses
S, F
+++
+++
+++
Herpes simplex virus
Y
Vesicle fluid
Parvovirus
Y
Amniotic fluid, liver tissue
Rubella
Y
++
Serum for antibody (IgM) detection
EYE (OCULAR DISEASE)
Adenoviruses
Y
++
Conjunctival swab or scraping
Herpes simplex virus
Y
Conjunctival swab or scraping
Varicella-zoster virus
Y
Conjunctival swab or scraping
POSTTRANSPLANTATION SYNDROME
Cytomegalovirus
Y
++
Blood (++++) shell vial and/or antigenemia; tissue (++++)
Epstein-Barr virus
Y
Serology, tissue (PCR) (EBV)
Human herpesvirus-6 (HH6)
Y
Serology, blood (PCR)
Herpes simplex
Y
Tissue (+++) virus
BK virus
Y
++++
Myocarditis, Pericarditis, and Pleurodynia
Coxsackie B
S, F
+++
++
Pericardial fluid (++++)
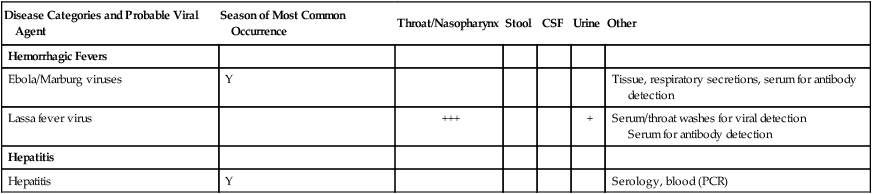
Hemorrhagic Fevers
Ebola/Marburg viruses
Y
Tissue, respiratory secretions, serum for antibody detection
Lassa fever virus
+++
+
Serum/throat washes for viral detection
Serum for antibody detection
Hepatitis
Hepatitis
Y
Serology, blood (PCR)
Specimen Processing
General Principles
Source
Specimen
Processing*
Cells for Detection of Common Viruses
Blood
Anticoagulated blood
Separate leukocytes (see Procedure 65-1)
PMK, HDF, HEp-2
Cerebrospinal fluid (CSF)
1 mL CSF
Inoculate directly
PMK, HDF, HEp-2
Stool or rectal swab
Pea-sized aliquot of feces
Place in 2 mL of viral transport medium vortex. Centrifuge at 1000× g for 15 min and use supernatant fluid for inoculum
PMK, HDF, HEp-2
Genital, skin
Vesicle fluid or scraping
Emulsify in viral transport medium
HDF
Miscellaneous
Swab, fluids
Emulsify in viral transport medium
Fluid, inoculate directly
PMK, HDF, HEp-2
Respiratory tract
Nasopharyngeal secretions, throat swab, respiratory tract washings, sputum
Dilute with viral transport medium
PMK, HDF, HEp-2
Tissue
Tissue in sterile container
Mince with sterile scalpel and scissors and gently grind. Prepare 20% suspension in viral transport medium. Centrifuge at 1000× g for 15 min and use supernatant fluid for inoculum.
PMK, HDF, HEp-2
Urine
Midstream specimen
Clear: Inoculate directly.
Turbid: Centrifuge at 1000× g for 15 min and use supernatant fluid for inocula.
HDF, HEp-2 (if adenovirus suspected) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Overview of the Methods and Strategies in Virology