Overview of Invasive Carcinoma Subtypes
Jesse K. McKenney, MD
Mahesha Vankalakunti, MD
Mahul B. Amin, MD
 Invasive urothelial carcinoma may show glandular differentiation that is morphologically identical to adenocarcinoma. The presence of a component of conventional urothelial carcinoma is distinctive. |
TERMINOLOGY
Definitions
Invasive urothelial carcinoma (UC) with morphology distinct from usual or typical pattern
CLINICAL IMPLICATIONS
Gender
Variants are most common in older men
Similar to urothelial carcinoma in general
Clinical Presentation
Hematuria most common
Treatment
Urothelial carcinoma variants are treated similarly to conventional urothelial carcinoma with some exceptions
Small cell carcinoma treated by separate chemotherapy regimen
Pure lymphoepithelioma-like carcinoma may be more responsive to chemotherapy
Micropapillary carcinoma may be treated surgically at low stage (pT1) in some centers
Urothelial carcinoma with squamous differentiation is less responsive to adjuvant therapy
Prognosis
Variant invasive urothelial carcinomas have poor prognosis
Generally present at high stage
Uncertain whether prognosis is worse than urothelial carcinoma of similar stage in some variants
MACROSCOPIC FINDINGS
General Features
Typically large infiltrative mass lesion
UC WITH ALTERNATIVE/ABERRANT DIFFERENTIATION
Microscopic Features
By definition, contains component of typical papillary, in situ, or invasive urothelial carcinoma at least focally
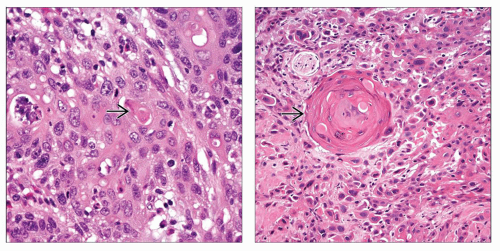
Squamous differentiation
Keratinization and intracellular bridges
May be focal or extensive
Glandular differentiation
Glandular component identical to adenocarcinoma
Trophoblastic differentiation
Scattered syncytiotrophoblasts within high-grade urothelial carcinoma
Rarely choriocarcinomatous differentiation
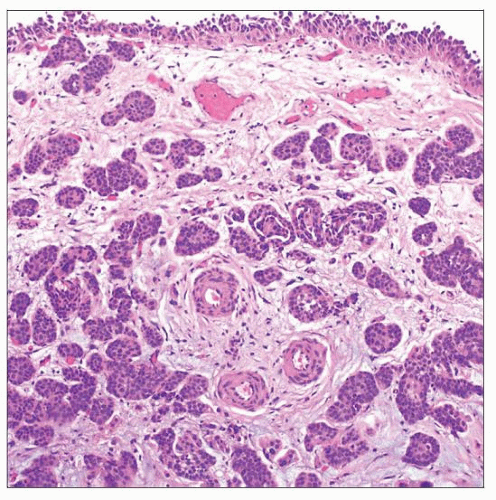
NESTED CARCINOMA
Microscopic Features
Nests of infiltrative tumor cells with relatively bland cytologic appearance
Irregular infiltrating border with lamina propria is characteristic
Muscularis propria is commonly involved
Tumor nests often have some degree of complex anastomosis at least focally
Invasion with surrounding retraction may be present focally
Generally show increasing levels of atypia toward deeper portions of tumor
May be admixed with urothelial carcinoma with small tubules
Differential Diagnosis
von Brunn nests
More rounded urothelial nests
Lobular configuration
Superficial location with sharp border at deep interface with lamina propria
Cystitis cystica/glandularis
More superficially located
Also has sharp border at interface with lamina propria
Nephrogenic adenoma
More tubular appearance
Prominent basement membranes may surround tubules
Lining epithelium may have “hobnail” appearance
Other admixed patterns may be present: Papillary, solid/diffuse, cystic
UC WITH SMALL TUBULES
Microscopic Features
Invasive carcinoma with small gland-like spaces lined by urothelial cells
No intracellular mucin
No columnar lining
May be admixed with nested variant
Same differential considerations as nested variant
MICROCYSTIC CARCINOMA
Microscopic Features
Dilated microcysts in invasive component
Microcysts may reach 1-2 mm in diameter
Urothelial lining
May be associated with nested variant
Differential Diagnosis
Urothelial carcinoma with glandular differentiation
Glandular component lined by columnar cells or has abundant intracytoplasmic mucin
Nephrogenic adenoma
More superficial location
No destructive invasion
Cystitis cystica/glandularis
Sharp linear base at junction with lamina propria
Müllerianosis
Endocervical, tubal, or endometrial-type glands
Bland cytologic features
PLASMACYTOID CARCINOMA
Microscopic Features
Malignant cells closely resemble plasma cells set in myxoid or loose edematous stroma
Eccentric nuclei
Abundant glassy eosinophilic cytoplasm
Clusters of neoplastic cells may be surrounded by retraction spaces
Concomitant conventional urothelial carcinoma may be admixed
Often have more extensive spread in abdominal cavity than other variants of urothelial carcinoma
Differential Diagnosis
Plasmacytoma and lymphoma
Plasmacytoid carcinoma may express CD138
Strong cytokeratin reactivity supports carcinoma
Evaluation of κ and λ ratio may be helpful
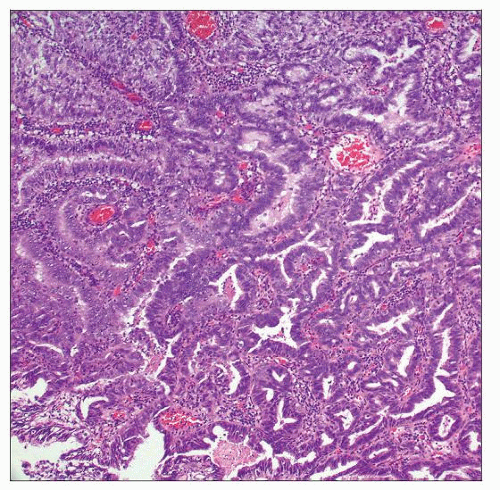
MICROPAPILLARY CARCINOMA
Microscopic Features
Small nests and papillae with surrounding retraction spaces
Resembles ovarian serous carcinoma
Confluent retraction spaces are characteristic
Multiple nests in same retraction space is common
Although nuclear grade is typically high, may also have relatively low-grade appearance
Most are muscle invasive with vascular invasion
CD31, CD34, and Podoplanin(D2-40) may help to distinguish true lymphatic invasion from retraction artifact
Immunohistochemically, tumor is reactive for EMA/MUC1, CK7, CK20
Immunoreactivity for HER2 and CA125 may also be seen
Differential Diagnosis
Ovarian serous carcinoma
Clinical/radiographic correlation is needed
Immunohistochemical expression of ER and WT1 is common in ovarian primary
Typical invasive urothelial carcinoma with stromal retraction
Larger nests
Does not typically show multiple small nests in same retraction space
Significant immunophenotypic overlap with micropapillary carcinoma: May also express EMA/MUC1, CA125, and HER2
In some cases, distinction may be very difficult
LYMPHOEPITHELIOMA-LIKE CARCINOMA
Microscopic Features
Resembles undifferentiated carcinomas of nasopharynx
Individual neoplastic cells arranged in syncytia with obscuring chronic inflammation
Cytoplasmic borders are most often indistinct
Inflammation consists of a mixture of polyclonal B and T lymphocytes, histiocytes, eosinophils, and plasma cells
Pure forms are reportedly more responsive to chemotherapy
Percentage of lymphoepithelioma-like areas should be reported
Differential Diagnosis
Lymphoma or chronic inflammation
CD45(LCA) reactivity in neoplastic cells
No cytokeratin-positive population
Small cell carcinoma
Neuroendocrine chromatin features
Cellular molding
High mitotic and apoptotic index
Coexpress cytokeratin and synaptophysin
May also express TTF-1
SMALL CELL CARCINOMA
Microscopic Features
Sheets and occasionally nests of cells with scant cytoplasm and high nuclear/cytoplasmic ratio
Chromatin is finely stippled, and nucleoli are inconspicuous
Geographic areas of necrosis, high mitotic rate, and areas of crush artifact are also frequent
Other subtypes of primary bladder carcinoma may be admixed
Urothelial carcinoma in situ, invasive urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, or sarcomatoid carcinoma
Identical pattern of allelic loss in small cell carcinoma and adjacent conventional urothelial carcinoma suggest shared lineage
Highly aggressive clinical behavior
Even focal small cell component should be reported
Differential Diagnosis
Metastatic small cell carcinoma
Histologically and immunophenotypically indistinguishable unless conventional urothelial carcinoma is present
CK7(+)/CK20(-) phenotype common
Both metastases and primary tumors may express TTF-1
Lymphoma
Express hematopoietic markers
Cytokeratin negative
Poorly differentiated urothelial carcinoma
Does not express synaptophysin or chromogranin
Rhabdomyosarcoma
May express synaptophysin
Nuclear myogenin reactivity diagnostic of skeletal muscle differentiation
SARCOMATOID UC
Microscopic Features
Neoplasms containing both epithelial and mesenchymal differentiation by morphology or immunohistochemistry
Epithelial component may be any subtype of bladder carcinoma
Urothelial carcinoma in situ, invasive urothelial carcinoma, squamous cell carcinoma, or adenocarcinoma
Mesenchymal component usually has high-grade spindle cell morphology
Heterologous elements may be present
Osteosarcoma, chondrosarcoma, and rhabdomyosarcoma
Immunohistochemical expression of HMCK(34βE12) and p63 in both epithelial and spindled component
Differential Diagnosis
Pseudosarcomatous myofibroblastic proliferation
Fine nuclear chromatin
Actin expression common
In contrast to carcinoma, cytokeratin expression limited to low molecular weight forms
Does not express p63
Subset expresses ALK1 by immunohistochemistry
Primary leiomyosarcoma
Expresses desmin and actin
In contrast to carcinoma, cytokeratin expression limited to low molecular weight forms
Up to 23% express p63
Other primary vesical sarcoma
No carcinomatous component or recent history of urothelial carcinoma
Nonepithelial immunophenotype
UNDIFFERENTIATED UC WITH OSTEOCLAST-LIKE GIANT CELLS
Microscopic Features
Prominent osteoclast-type giant cells are seen in rare undifferentiated carcinomas
Giant cells are histiocytic in origin
Background spindled and mononuclear cells are cytokeratin positive
UC WITH RHABDOID FEATURES
Microscopic Features
Very rare morphologic subtype
Neoplastic cells with large vesicular nuclei, prominent nucleoli, and eosinophilic cytoplasmic inclusions
Resembles malignant extrarenal rhabdoid tumor
Does not have deletion of INI1 at 22q11
Usually adult tumor, unlike malignant extrarenal rhabdoid tumor
Very aggressive clinical course
UC WITH MYXOID STROMA
Microscopic Features
Typical urothelial carcinoma almost always present
Prominent myxoid stroma
Proportion of tumor highly variable
Neoplastic cells may “float” in myxoid matrix in aggregates or chains
Small round cells with eosinophilic cytoplasm are common
UC WITH CLEAR CYTOPLASM (GLYCOGEN RICH)
Microscopic Features
Abundant clear cytoplasm secondary to glycogen accumulation
Typically focal pattern in otherwise typical urothelial carcinoma
Differential Diagnosis
Renal cell carcinoma
Obvious renal mass present
Expression of pax-2 may be seen
Clear cell adenocarcinoma, primary or gynecologic
Distinct mixed tubulocystic and papillary pattern with “hobnail” cells typical
UC WITH LIPOID FEATURES (LIPID-RICH/LIPID CELL)
Microscopic Features
Rare urothelial carcinomas have foci with intracellular lipid
Closely resemble lipoblasts
Most admixed with typical urothelial carcinoma
Maintain cytokeratin immunoreactivity, even in lipid-rich cells
Differential Diagnosis
Primary liposarcoma
Lack component of typical urothelial carcinoma
Epithelioid variant of pleomorphic liposarcoma is close mimic that may express keratin
Sarcomatoid urothelial carcinoma with heterologous liposarcoma
Usually has pleomorphic spindled component
Lipoblasts do not express cytokeratin
Other heterologous components may be admixed
Signet ring cell adenocarcinoma
Smaller cells with single intracytoplasmic vacuoles
Often infiltrate as individual cells
LARGE CELL UNDIFFERENTIATED CARCINOMA
Microscopic Features
Poorly differentiated pleomorphic carcinoma without histologic features typical of urothelial carcinoma
Differential Diagnosis
Lymphoma
Expresses hematopoietic markers
Secondary carcinoma from another anatomic site
Requires clinical correlation
Melanoma
Expresses S100
DIFFERENTIAL DIAGNOSIS
Secondary Carcinomas from Nonbladder Sites
Variant morphologic patterns of urothelial carcinoma may suggest nonbladder primary
Most urothelial carcinoma variants maintain urothelial immunophenotype
CK7 and CK20 coexpression common
Express HMCK(34βE12)
Nuclear p63 reactivity
DIAGNOSTIC CHECKLIST
Pathologic Interpretation Pearls
Variant morphology carcinoma: Primary carcinoma involving bladder and not conforming to morphology of typical urothelial carcinoma
Variant histology must be documented, including percentage, if not pure in histology
Variant histology may present at metastatic site; facilitates association with bladder primary
Variant histology may have diagnostic, prognostic, or therapeutic significance
Metastatic carcinoma or carcinoma secondarily involving bladder must be ruled out in all cases
SELECTED REFERENCES
1. Amin MB: Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 22 Suppl 2:S96-S118, 2009
2. Nigwekar P et al: Plasmacytoid urothelial carcinoma: detailed analysis of morphology with clinicopathologic correlation in 17 cases. Am J Surg Pathol. 33(3):417-24, 2009
3. Drew PA et al: The nested variant of transitional cell carcinoma: an aggressive neoplasm with innocuous histology. Mod Pathol. 9(10):989-94, 1996
4. Amin MB et al: Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 18(12):1224-32, 1994
5. Young RH et al: Unusual forms of carcinoma of the urinary bladder. Hum Pathol. 22(10):948-65, 1991
Image Gallery
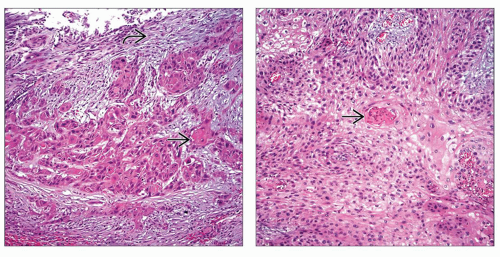
Carcinoma with Squamous and Glandular Differentiation
 (Left) Focal keratin formation
 is seen in this example of urothelial carcinoma with squamous differentiation. (Right) Keratin pearl formation is seen in this example of urothelial carcinoma with squamous differentiation. (Right) Keratin pearl formation  is the prototypical feature of squamous differentiation. In contrast to primary squamous cell carcinoma, urothelial carcinoma with squamous differentiation has areas of conventional papillary, invasive, or in situ urothelial carcinoma. In addition, primary squamous cell carcinoma arises in a background of squamous metaplasia/dysplasia. is the prototypical feature of squamous differentiation. In contrast to primary squamous cell carcinoma, urothelial carcinoma with squamous differentiation has areas of conventional papillary, invasive, or in situ urothelial carcinoma. In addition, primary squamous cell carcinoma arises in a background of squamous metaplasia/dysplasia.Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|