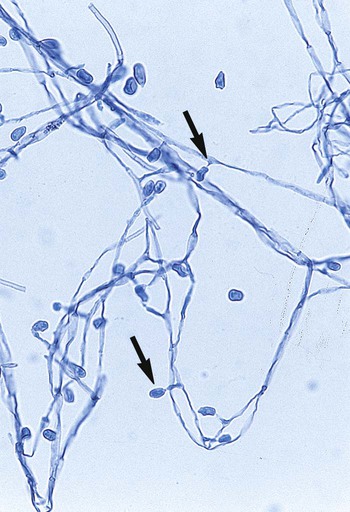
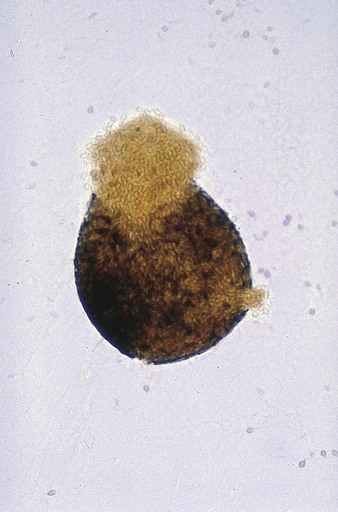
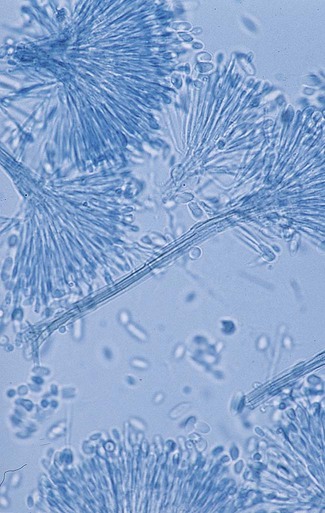
Chapter 59 1. Define the terms mycology, saprophytic, dermatophyte, and polymorphic, dimorphic, and thermally dimorphic fungi. 2. Define and differentiate superficial, cutaneous, subcutaneous, and systemic mycoses, including the tissues involved. 3. Differentiate the colonial morphology of yeasts and filamentous fungi (molds). 4. Define and differentiate anamorph, teleomorph, and synanamorph. 5. Describe three ways in which fungi reproduce. 6. List the media that should be used for optimal recovery of fungi, including their incubation requirements. 7. List the common antibacterial agents used in fungal media. 8. Explain and differentiate the characteristic colonial morphology of fungi, including topography (rugose, umbonate, verrucose), texture (cottony, velvety, glabrous, granular, wooly) and surface described (front, reverse). 9. Describe and differentiate the sexual and asexual reproduction of the Ascomycota. 10. Define and differentiate rapid, intermediate, and slow growth rates with regard to fungal reproduction and cultivation. 11. Describe the proper method of specimen collection for fungal cultures, including collection site, acceptability, processing, transport, and storage. 12. Give the advantages and disadvantages of using screw-capped culture tubes, compared with agar plates, in the laboratory. 13. Describe the chemical principle and methodologies used to identify fungi, including calcofluor white–potassium hydroxide preparations, hair perforation, cellophane (Scotch) tape preparations, saline/wet mounts, lactophenol cotton blue, potassium hydroxide, Gram stain, India ink, modified acid-fast stain, periodic acid-Schiff stain (PAS), Wright’s stain, Papanicolaou stain, Grocott’s methenamine silver (GMS), hematoxylin and eosin (H&E) stain, Masson-Fontana stain, tease mount and microslide culture. Fungi seen in the clinical laboratory generally can be categorized into two groups based on the appearance of the colonies formed. The yeasts produce moist, creamy, opaque or pasty colonies on media, whereas the filamentous fungi or molds (see Chapters 60 and 61) produce fluffy, cottony, woolly, or powdery colonies. Several systemic fungal pathogens exhibit either a yeast (or yeastlike) phase, and filamentous forms are referred to as dimorphic. When dimorphism is temperature dependent, the fungi are designated as thermally dimorphic. In general, these fungi produce a mold form at 25° to 30°C and a yeast form at 35° to 37°C under certain circumstances. The medically important dimorphic fungi are Histoplasma capsulatum, Blastomyces dermatitidis, C. immitis, Paracoccidioides brasiliensis, Sporothrix schenckii, and Penicillium marneffei (see Chapter 60). C. immitis is not thermally dimorphic. Additionally, some of the medically important yeasts, particularly the Candida species, may produce yeasts forms, pseudohyphae, and/or true hyphae (see Chapter 62). Fungi that have more than one independent form or spore stage in their life cycle are called polymorphic fungi. The polymorphic features of this group of organisms are not temperature dependent. • Ergosterol in the cell membrane • Reproduction by means of spores, produced asexually or sexually • Lack of susceptibility to antibacterial antibiotics • Saprophytic nature (derive nutrition from organic materials) The Ascomycota include many fungi that reproduce asexually by the formation of conidia (asexual spores) and sexually by the production of ascospores. The filamentous ascomycetes are ubiquitous in nature, and all produce true septate hyphae. All exhibit a sexual form (teleomorph) but also exist in an asexual form (anamorph). Fungi that have different asexual forms of the same fungus are called synanomorphs. In general, the anamorphic form correlates well with the teleomorphic classification. However, different anamorphic forms may have the same teleomorphic form. For example, Pseudallescheria boydii (Figure 59-1), in addition to having the Scedosporium apiospermum anamorph (Figure 59-2), may exhibit a Graphium anamorph (Figure 59-3). The latter anamorph may be seen with several other fungi. The botanic taxonomic schema for grouping the fungi has little value in a clinical microbiology laboratory. Table 59-1 is a simplified taxonomic schema illustrating the major groups of fungi. TABLE 59-1 Phylogenetic Position of Medically Significant Fungi *When the sexual form is known. †Most commonly encountered as causes of infection. Modified from the Catalogue of Life. November 20, 2012. http://www.catalogueoflife.org/col/browse/classification and Hibbet DS, Binder M, Bischoff JF, et al. A higher-level of classification of the fungi, Mycological Research, 509-547, 2007. Some fungi cause infections that are confined to the subcutaneous tissue without dissemination to distant sites. Examples of subcutaneous infections include chromoblastomycosis, mycetoma, and phaeohyphomycotic cysts (see Chapter 61). Classification by type of infection allows the clinician to attempt to categorize organisms in a logical fashion into groups having clinical relevance. Table 59-2 presents an example of a clinical classification of infections and their etiologic agents that is useful to clinicians. TABLE 59-2 General Clinical Classification of Pathogenic Fungi *Virtually any fungus may cause disease in a profoundly immunocompromised host. To assist individuals working in clinical microbiology laboratories with the identification of clinically important fungi, Koneman and Roberts1 have suggested a practical working scheme designed to do the following: • Assist with the recognition of fungi most commonly encountered in clinical specimens • Assist with the recognition of fungi recovered on culture media that are strictly pathogenic fungi • Provide a pathway that allows an identification to be made based on a few colonial and microscopic features Table 59-3 presents these features. However, the table includes only organisms commonly seen in the clinical laboratory. With practice, most laboratorians should be able to recognize these on a day-to-day basis. For other, less commonly encountered fungi, the microbiologist must use a variety of texts that have photomicrographs, which can aid identification. TABLE 59-3 Most Commonly Encountered Fungi of Clinical Laboratory Importance: a Practical Working Schema *Rudimentary hyphae may be present. From Koneman EW, Roberts GD: Practical laboratory mycology, ed 3, Baltimore, 1985, Williams & Wilkins. Use of the identification scheme just described requires examination of the fungal culture for the presence, absence, and number of septa. If the hyphae appear to be broad and predominantly nonseptate (i.e., cells are not separated by a septum or wall), zygomycetes should be considered. If the hyphae are septate, they must be examined further for the presence or absence of pigmentation. If a dark pigment is present in the hyphae, the organism is considered to be dematiaceous, and the conidia are then examined for their morphologic features and their arrangement on the hyphae. If the hyphae are nonpigmented, they are considered to be hyaline. The fungi are then examined for the type and the arrangement of the conidia produced. The molds are identified by recognition of their characteristic microscopic features (see Table 59-3). Murray2 has developed an expanded morphologic classification of medically important fungi based on general microscopic features and colonial morphology. The color pigmentation of colonies is presented as a useful diagnostic feature (Box 59-1). • The organism’s size (with inhalation, the organism must be small enough to reach the alveoli) • The organism’s ability to grow at 37°C at a neutral pH • Conversion of the dimorphic fungi from the mycelial form into the corresponding yeast or spherule form in the host Most of the fungi exist in environmental niches as saprophytic organisms (Table 59-4). Perhaps the fungi that cause disease in humans have developed various mechanisms that allow them to establish disease in the human host. Table 59-5 describes the known or speculative virulence factors of the fungi known to be pathogenic for humans. TABLE 59-4 GI, Gastrointestinal; GU, genitourinary. *Possibly the conidia of the teleomorphic stage (Filobasidiella neoformans). TABLE 59-5 Virulence Factors of Medically Important Fungi
Overview of Fungal Identification Methods and Strategies
General Features of the Fungi
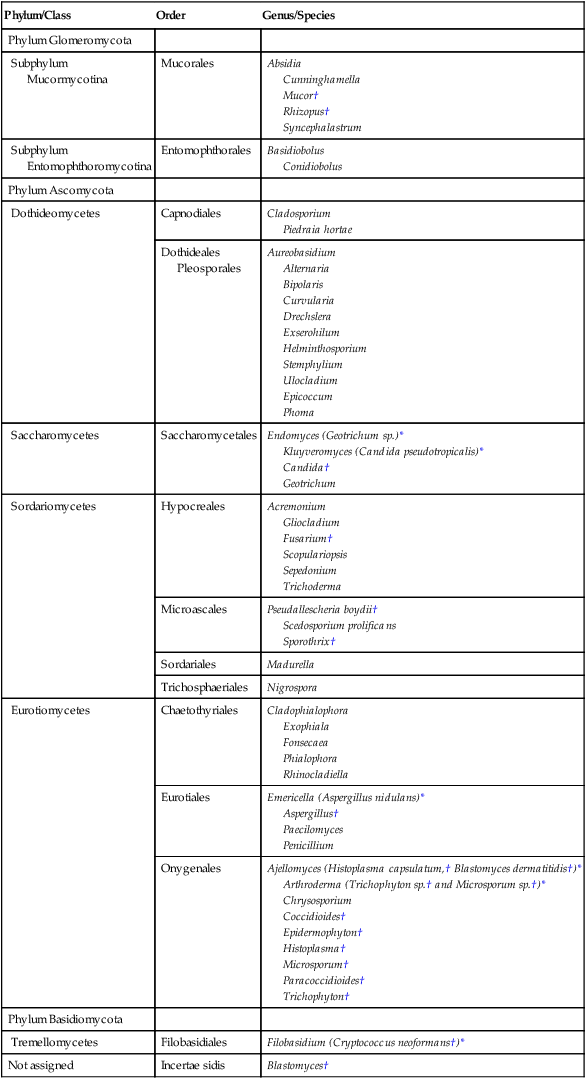
Taxonomy of the Fungi
Clinical Classification of the Fungi
Phylum/Class
Order
Genus/Species
Phylum Glomeromycota
Subphylum
Mucormycotina
Mucorales
Absidia
Cunninghamella
Mucor†
Rhizopus†
Syncephalastrum
Subphylum
Entomophthoromycotina
Entomophthorales
Basidiobolus
Conidiobolus
Phylum Ascomycota
Dothideomycetes
Capnodiales
Cladosporium
Piedraia hortae
Dothideales
Pleosporales
Aureobasidium
Alternaria
Bipolaris
Curvularia
Drechslera
Exserohilum
Helminthosporium
Stemphylium
Ulocladium
Epicoccum
Phoma
Saccharomycetes
Saccharomycetales
Endomyces (Geotrichum sp.)*
Kluyveromyces (Candida pseudotropicalis)*
Candida†
Geotrichum
Sordariomycetes
Hypocreales
Acremonium
Gliocladium
Fusarium†
Scopulariopsis
Sepedonium
Trichoderma
Microascales
Pseudallescheria boydii†
Scedosporium prolificans
Sporothrix†
Sordariales
Madurella
Trichosphaeriales
Nigrospora
Eurotiomycetes
Chaetothyriales
Cladophialophora
Exophiala
Fonsecaea
Phialophora
Rhinocladiella
Eurotiales
Emericella (Aspergillus nidulans)*
Aspergillus†
Paecilomyces
Penicillium
Onygenales
Ajellomyces (Histoplasma capsulatum,† Blastomyces dermatitidis†)*
Arthroderma (Trichophyton sp.† and Microsporum sp.†)*
Chrysosporium
Coccidioides†
Epidermophyton†
Histoplasma†
Microsporum†
Paracoccidioides†
Trichophyton†
Phylum Basidiomycota
Tremellomycetes
Filobasidiales
Filobasidium (Cryptococcus neoformans†)*
Not assigned
Incertae sidis
Blastomyces†
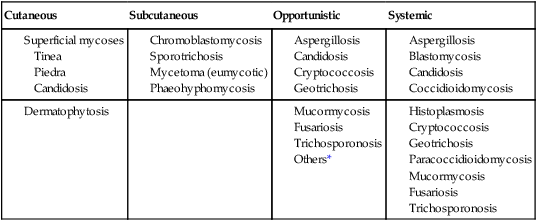
Cutaneous
Subcutaneous
Opportunistic
Systemic
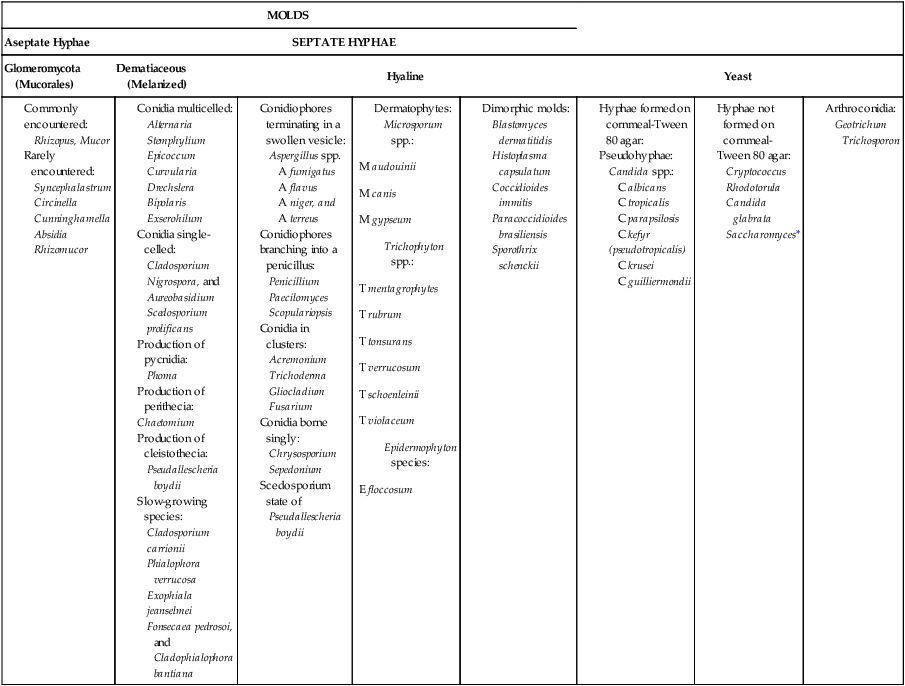
Practical Working Schema
MOLDS
Aseptate Hyphae
SEPTATE HYPHAE
Glomeromycota (Mucorales)
Dematiaceous (Melanized)
Hyaline
Yeast
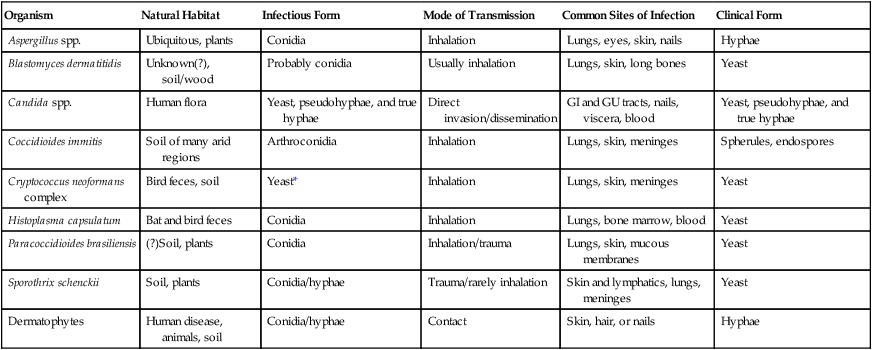
Pathogeneis and Spectrum of Disease
Organism
Natural Habitat
Infectious Form
Mode of Transmission
Common Sites of Infection
Clinical Form
Aspergillus spp.
Ubiquitous, plants
Conidia
Inhalation
Lungs, eyes, skin, nails
Hyphae
Blastomyces dermatitidis
Unknown(?), soil/wood
Probably conidia
Usually inhalation
Lungs, skin, long bones
Yeast
Candida spp.
Human flora
Yeast, pseudohyphae, and true hyphae
Direct invasion/dissemination
GI and GU tracts, nails, viscera, blood
Yeast, pseudohyphae, and true hyphae
Coccidioides immitis
Soil of many arid regions
Arthroconidia
Inhalation
Lungs, skin, meninges
Spherules, endospores
Cryptococcus neoformans complex
Bird feces, soil
Yeast*
Inhalation
Lungs, skin, meninges
Yeast
Histoplasma capsulatum
Bat and bird feces
Conidia
Inhalation
Lungs, bone marrow, blood
Yeast
Paracoccidioides brasiliensis
(?)Soil, plants
Conidia
Inhalation/trauma
Lungs, skin, mucous membranes
Yeast
Sporothrix schenckii
Soil, plants
Conidia/hyphae
Trauma/rarely inhalation
Skin and lymphatics, lungs, meninges
Yeast
Dermatophytes
Human disease, animals, soil
Conidia/hyphae
Contact
Skin, hair, or nails
Hyphae
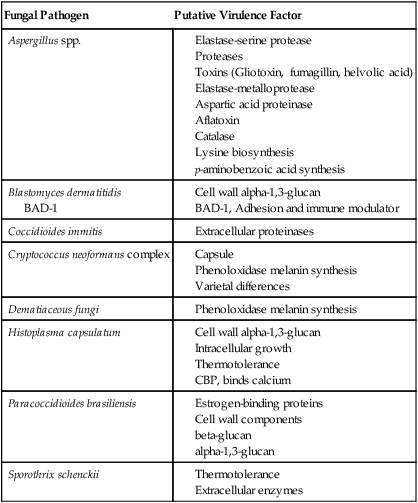
Fungal Pathogen
Putative Virulence Factor
Aspergillus spp.
Blastomyces dermatitidis
BAD-1
Coccidioides immitis
Cryptococcus neoformans complex
Dematiaceous fungi
Histoplasma capsulatum
Paracoccidioides brasiliensis
Sporothrix schenckii
Basicmedical Key
Fastest Basicmedical Insight Engine