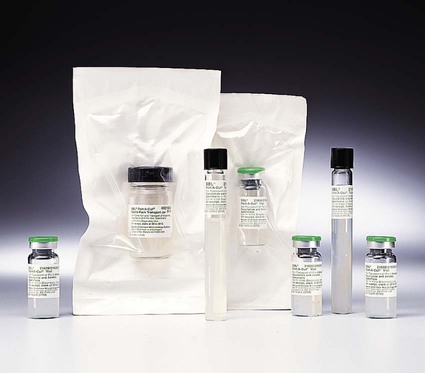
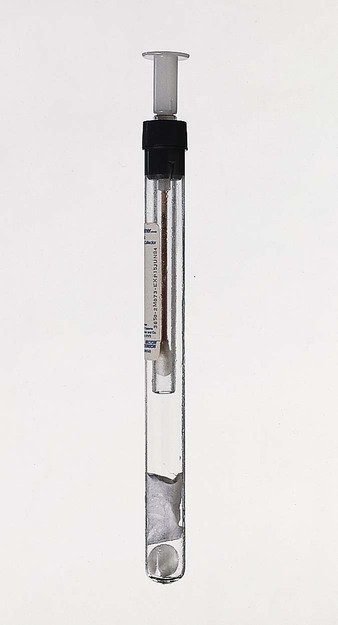
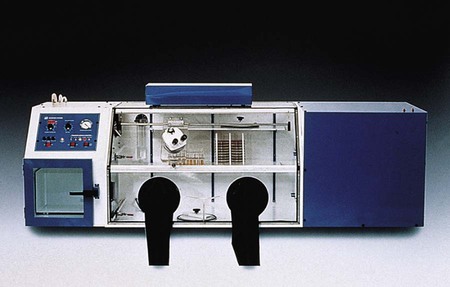
Chapter 41 This chapter provides an overview of the methods used to identify anaerobic microorganisms. The detailed technical procedures discussed should be used in conjunction with specifics provided in Chapter 42 to develop a clear understanding of the full process, from specimen collection to identification. However, readers should consider the following general objectives for the information and methods provided. 1. State the specific diagnostic purpose for the test methodology. 2. Briefly describe the test principle associated with the test methodology. 3. Outline limitations and describe a process for trouble-shooting or reporting results if a test result is equivocal or indistinguishable. 4. State the appropriate quality control organisms and results used with each testing procedure. 5. Define and differentiate obligate (strict), moderate, facultative, and aerotolerant anaerobes. 6. List suitable specimens for isolation of anaerobic bacteria and characteristics of these specimens that might suggest the presence of an anaerobic infection. 7. Explain the proper techniques for collecting, transporting, and processing clinical specimens for anaerobic bacteriology. 8. Explain the use of antigen detection methodologies in the diagnosis of anaerobic infections. 9. List the media used for cultivation of anaerobic bacteria. 10. Describe the appropriate incubation conditions for cultivation of anaerobic bacteria. 11. Describe the procedures for the identification of and antibiotic susceptibility testing for anaerobic bacteria. The organisms described in this chapter and in Chapter 42 usually do not grow in the presence of oxygen (O2); they are obligate, or strict, anaerobes (0% O2). Obligate anaerobes are killed upon brief exposure (less than a few minutes) to atmospheric oxygen. Obligate anaerobes include Prevotella sp., Fusobacterium sp., and Bacteroides spp. These chapters also include some aerotolerant organisms (5% O2), such as Actinomyces spp., Bifidobacterium spp., and Clostridium spp., which are capable of growth in the presence of either reduced or atmospheric oxygen but grow best under anaerobic conditions. Finally, facultative anaerobes do not require atmospheric oxygen but are capable of growth in oxygen and anaerobic environments. The importance of proper collection and transport of specimens for anaerobic culture cannot be overemphasized. Because indigenous anaerobes are often present in large numbers as normal flora on mucosal surfaces, even minimal contamination of a specimen can produce misleading results. Box 41-1 shows the specimens acceptable for anaerobic culture; Box 41-2 presents specimens that are likely to be contaminated and therefore are unacceptable for anaerobic culture. In general, material for anaerobic culture is best obtained by tissue biopsy or by aspiration using a needle and syringe. Use of swabs is a poor alternative because of excessive exposure of the specimen to the deleterious effects of drying, the possibility of contamination during collection, and the easy retention of microorganisms in the fibers of the swab. If a swab must be used, it should be from an oxygen-free transport system. Three kinds of anaerobic transport systems are shown in Figures 41-1 to 41-3. Figure 41-1 shows is a rubber-stoppered collection vial containing an agar indicator system. The vial is gassed out with oxygen-free carbon dioxide (CO2) or nitrogen. The specimen (pus, body fluid, or other liquid material) is injected through the rubber stopper after all air has been expelled from the syringe and needle. If only a swab specimen can be obtained, a special collection device with an oxygen-free atmosphere is required (see Figure 41-2). When the swab is reinserted, care must be taken not to tip the container, which would cause the oxygen-free CO2 or nitrogen to spill out and be displaced by ambient air. A tissue specimen can be immersed in a small amount of liquid to prevent it from drying and then placed in an anaerobic pouch (see Figure 41-3). All specimens should be held at room temperature pending processing in the laboratory, because refrigeration can oxygenate the specimen. Table 41-1 presents the cellular morphology seen with Gram staining of common anaerobes. TABLE 41-1 Ana BAP, Anaerobic blood agar plate; BBE, Bacteroides bile esculin agar; CCFA, cycloserine cefoxitin fructose agar; LKV, laked kanamycin-vancomycin blood agar; UV, ultraviolet. *Typical Gram stain appearance is seen from broth (thioglycollate or peptone-yeast-glucose). The most frequently used system for creating an anaerobic atmosphere is the anaerobe jar. Anaerobe jars are available commercially from several companies. For example, the GasPak (Figure 41-4) is made by Becton Dickinson (Sparks, Maryland); other companies that produce these devices include EM Diagnostic Systems (Gibbstown, New Jersey) and Oxoid U.S.A. (Columbus, Maryland). All of these systems use a clear, heavy plastic jar with a lid that is clamped down to make it airtight. Anaerobic conditions can be set up by two methods. The easiest method uses a commercially available envelope containing a hydrogen and CO2 generator that is activated either by adding water (GasPak) or by the moisture on the agar plates (EM Diagnostic Systems and Oxoid USA). The production of heat within a few minutes (detected by touching the top of the jar) and subsequent development of moisture on the walls of the jar are indications that the catalyst and generator envelope are functioning properly. Reduced conditions are achieved in 1 to 2 hours, although the methylene blue or resazurin indicators take longer to decolorize. Alternatively, the “evacuation-replacement” method can be used. Air is removed from the sealed jar by drawing a vacuum of 25 inches (62.5 cm) of mercury. This process is repeated two times, with the jar being filled with an oxygen-free gas, such as nitrogen, between evacuations. The final fill of the jar is made with a gas mixture containing 80% to 90% nitrogen, 5% to 10% hydrogen, and 5% to 10% CO2. Many anaerobes require CO2 for maximal growth. The atmosphere in the jars is monitored using an indicator to check anaerobiosis. Anaerobe bags or pouches are useful for laboratories processing small numbers of anaerobic specimens. A widely used anaerobic pouch, the GasPak Pouch, is shown in Figure 41-3. Besides specimen transport, the pouch also can be used to incubate one or two agar plates. Anaerobic chambers, or glove boxes, are made of molded or flexible clear plastic. The flexible clear plastic chambers are the most widely used type. Specimens and other materials are placed in the chamber through an air lock. The technologist uses gloves (Forma Scientific, Marietta, Ohio) or sleeves (Sheldon Manufacturing, Cornelius, Oregon), to form airtight seals around the arms (Figure 41-5). Media stored in the chamber are kept oxygen free, and all work on a specimen, from inoculation through workup, is performed under anaerobic conditions. A gas mixture of 5% CO2, 10% hydrogen, and 85% nitrogen, plus a palladium catalyst, maintain the anaerobic environment inside the chamber. Initial processing of anaerobic specimens involves inoculation of appropriate media. Table 41-2 lists commonly used anaerobic media. Primary plates should be freshly prepared or used within 2 weeks of preparation. Plates stored for longer periods accumulate peroxides and become dehydrated; this results in growth inhibition. Reduction of media in an anaerobic environment eliminates dissolved oxygen but has no effect on the peroxides. Prereduced, anaerobically sterilized (PRAS) media are produced, packaged, shipped, and stored under anaerobic conditions. They are commercially available from Anaerobe Systems (Morgan Hill, California) (Figure 41-6) and have an extended shelf life of up to 6 months. TABLE 41-2
Overview and General Considerations
General Characteristics
Specimen Collection and Transport
Direct Detection Methods
Antigen Detection
Gram Staining
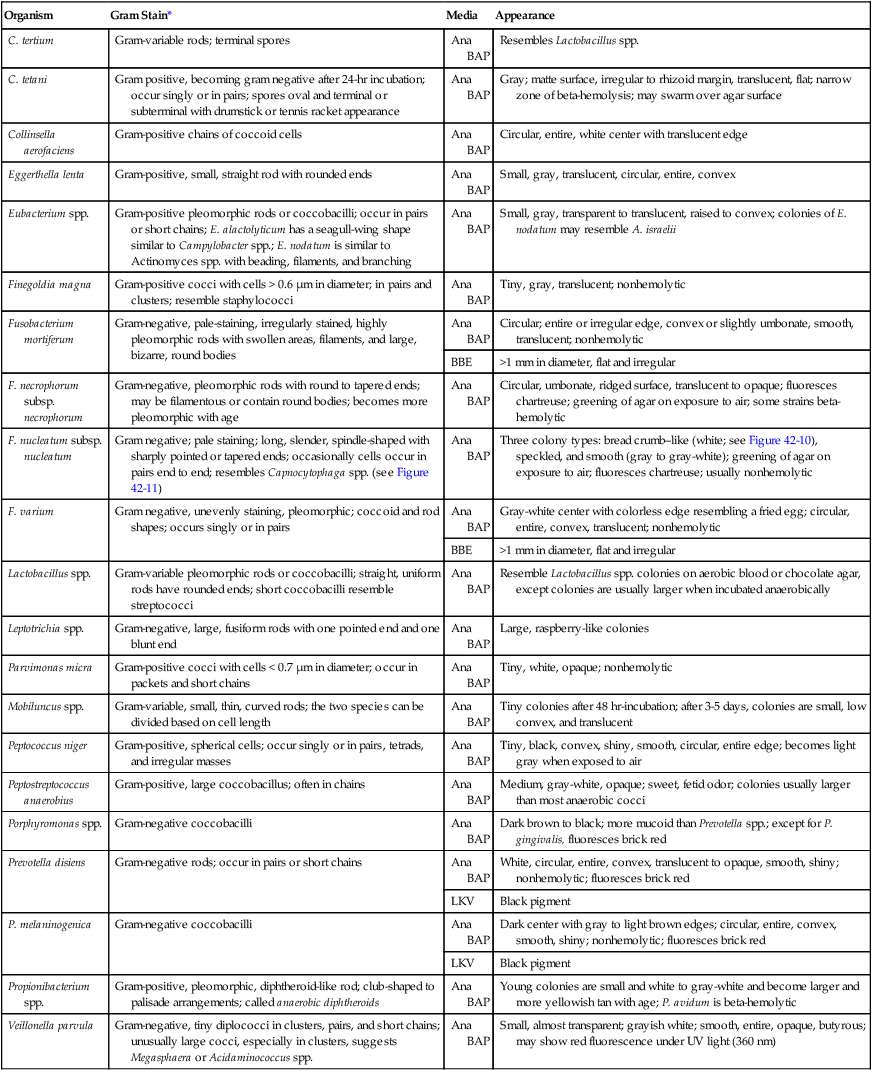
Organism
Gram Stain*
Media
Appearance
Actinomyces spp.
Gram-positive, branching, beaded or banded, thin, filamentous rods
Ana BAP
Colonies of most species are small, smooth, flat, convex, gray-white, translucent, with entire margins; colonies of A. israelii and A. gerencseriae are white, opaque, and may resemble a “molar tooth”; A. odontolyticus turns red after several days in ambient air and may be beta-hemolytic
Anaerococcus spp.
Gram-positive cocci arranged in short chains or tetrads
Ana BAP
Small, white, translucent, smooth
Atopobium spp.
Elongated gram-positive cocci; occur singly, in pairs, or in short chains
Ana BAP
Resemble lactobacilli
Bacteroides distasonis
Gram-negative, straight rods with rounded ends; occur singly or in pairs
Ana BAP
Gray-white, circular, entire, convex, smooth, translucent to opaque; nonhemolytic
BBE
At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone
B. fragilis
Gram-negative, pale-staining, pleomorphic rods with rounded ends; occur singly or in pairs; cells often described as resembling a safety pin (see Figure 42-4)
Ana BAP
White to gray, circular, entire, convex, translucent to semiopaque; nonhemolytic (see Figure 42-5)
BBE
At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone (see Figure 42-6)
B. ovatus
Gram-negative, ovoid rods with rounded ends; occur singly or in pairs
Ana BAP
Pale buff, circular, entire, convex, semiopaque; often mucoid; nonhemolytic
BBE
At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone
B. thetaiotaomicron
Gram-negative, irregularly staining, pleomorphic rods with rounded ends; occur singly or in pairs
Ana BAP
White, circular, entire, convex, semiopaque, shiny, punctiform; nonhemolytic
BBE
At 48 hr, colonies are >1 mm, circular, entire, raised, and either (1) low convex, dark gray, friable, and surrounded by a dark gray zone (esculin hydrolysis) and sometimes a precipitate (bile) or (2) glistening, convex, light to dark gray, and surrounded by a gray zone
B. ureolyticus
Gram-negative, pale-staining, thin, delicate rods with rounded ends; some curved
Ana BAP
Small, translucent or transparent; may produce greening of agar on exposure to air; colonies corrode (pit) the agar (see Figure 42-9) or may be smooth and convex or spreading
B. vulgatus
Gram-negative, pleomorphic rods with rounded ends; occur singly, in pairs, or in short chains; swellings or vacuoles may be seen
Ana BAP
Gray, circular, entire, convex, semiopaque; nonhemolytic
BBE
At 48 hr, colonies are >1 mm, circular, entire, raised, glistening, convex, light to dark gray but with no gray zone (esculin not hydrolyzed)
Bifidobacterium spp.
Gram-positive diphtheroid; coccoid or thin, pointed shape; or larger, highly irregular, curved rods with branching; rods terminate in clubs or thick, bifurcated (forked) ends (“dog bones”)
Ana BAP
Small, white, convex, shiny, with irregular edge
Bilophila wadsworthia
Gram-negative, pale-staining, delicate rods
Ana BAP
Small, translucent
BBE
Grows at 3-5 days; colonies are usually gray with a black center because of production of hydrogen sulfide (H2S); black center may disappear after exposure to air
Clostridium botulinum
Gram-positive, straight rods; occur singly or in pairs; spores usually subterminal and resemble a tennis racket
Ana BAP
Gray-white; circular to irregular; usually beta-hemolytic
C. clostridioforme
Gram-positive rod that stains gram negative; long, thin rods; spores usually not seen; elongated football shape with cells often in pairs
Ana BAP
Small, convex, entire edge; nonhemolytic
C. difficile
Gram-positive straight rods; may produce chains of up to six cells aligned end to end; spores oval and subterminal
Ana BAP
Large, white, circular, matte to glossy, convex, opaque; nonhemolytic; horse stable odor; fluoresces yellow-green
CCFA
Yellow, ground-glass colony (see Figure 42-2)
C. perfringens
Gram-variable straight rods with blunt ends; occur singly or in pairs; spores seldom seen but if present are large and central to subterminal, oval, and swell cell; large boxcar shapes (see Figure 44-6)
Ana BAP
Gray to grayish yellow; circular, glossy, dome shaped, entire, translucent; double zone of beta-hemolysis (see Figure 42-3)
C. ramosum
Gram-variable straight or curved rods; spores rarely seen but are round and terminal; more slender and longer than C. perfringens
Ana BAP
Small, gray-white to colorless; circular to slightly irregular, smooth, translucent or semiopaque; nonhemolytic
C. septicum
Gram positive in young cultures but becomes gram negative with age; stains unevenly; straight or curved rods; occur singly or in pairs; spores subterminal, and oval and swell cells
Ana BAP
Gray; circular, glossy, translucent; markedly irregular to rhizoid margins resembling a “Medusa head”; beta-hemolytic; swarms over entire agar surface in less than 24 hr
C. sordellii
Gram-positive rods; subterminal spores
Ana BAP
Large colony with irregular edge
C. sporogenes
Gram-positive rods; subterminal spores
Ana BAP
Colonies firmly adhere to agar; may swarm over agar surface
C. tertium
Gram-variable rods; terminal spores
Ana BAP
Resembles Lactobacillus spp.
C. tetani
Gram positive, becoming gram negative after 24-hr incubation; occur singly or in pairs; spores oval and terminal or subterminal with drumstick or tennis racket appearance
Ana BAP
Gray; matte surface, irregular to rhizoid margin, translucent, flat; narrow zone of beta-hemolysis; may swarm over agar surface
Collinsella aerofaciens
Gram-positive chains of coccoid cells
Ana BAP
Circular, entire, white center with translucent edge
Eggerthella lenta
Gram-positive, small, straight rod with rounded ends
Ana BAP
Small, gray, translucent, circular, entire, convex
Eubacterium spp.
Gram-positive pleomorphic rods or coccobacilli; occur in pairs or short chains; E. alactolyticum has a seagull-wing shape similar to Campylobacter spp.; E. nodatum is similar to Actinomyces spp. with beading, filaments, and branching
Ana BAP
Small, gray, transparent to translucent, raised to convex; colonies of E. nodatum may resemble A. israelii
Finegoldia magna
Gram-positive cocci with cells > 0.6 µm in diameter; in pairs and clusters; resemble staphylococci
Ana BAP
Tiny, gray, translucent; nonhemolytic
Fusobacterium mortiferum
Gram-negative, pale-staining, irregularly stained, highly pleomorphic rods with swollen areas, filaments, and large, bizarre, round bodies
Ana BAP
Circular; entire or irregular edge, convex or slightly umbonate, smooth, translucent; nonhemolytic
BBE
>1 mm in diameter, flat and irregular
F. necrophorum subsp. necrophorum
Gram-negative, pleomorphic rods with round to tapered ends; may be filamentous or contain round bodies; becomes more pleomorphic with age
Ana BAP
Circular, umbonate, ridged surface, translucent to opaque; fluoresces chartreuse; greening of agar on exposure to air; some strains beta-hemolytic
F. nucleatum subsp. nucleatum
Gram negative; pale staining; long, slender, spindle-shaped with sharply pointed or tapered ends; occasionally cells occur in pairs end to end; resembles Capnocytophaga spp. (see Figure 42-11)
Ana BAP
Three colony types: bread crumb–like (white; see Figure 42-10), speckled, and smooth (gray to gray-white); greening of agar on exposure to air; fluoresces chartreuse; usually nonhemolytic
F. varium
Gram negative, unevenly staining, pleomorphic; coccoid and rod shapes; occurs singly or in pairs
Ana BAP
Gray-white center with colorless edge resembling a fried egg; circular, entire, convex, translucent; nonhemolytic
BBE
>1 mm in diameter, flat and irregular
Lactobacillus spp.
Gram-variable pleomorphic rods or coccobacilli; straight, uniform rods have rounded ends; short coccobacilli resemble streptococci
Ana BAP
Resemble Lactobacillus spp. colonies on aerobic blood or chocolate agar, except colonies are usually larger when incubated anaerobically
Leptotrichia spp.
Gram-negative, large, fusiform rods with one pointed end and one blunt end
Ana BAP
Large, raspberry-like colonies
Parvimonas micra
Gram-positive cocci with cells < 0.7 µm in diameter; occur in packets and short chains
Ana BAP
Tiny, white, opaque; nonhemolytic
Mobiluncus spp.
Gram-variable, small, thin, curved rods; the two species can be divided based on cell length
Ana BAP
Tiny colonies after 48 hr-incubation; after 3-5 days, colonies are small, low convex, and translucent
Peptococcus niger
Gram-positive, spherical cells; occur singly or in pairs, tetrads, and irregular masses
Ana BAP
Tiny, black, convex, shiny, smooth, circular, entire edge; becomes light gray when exposed to air
Peptostreptococcus anaerobius
Gram-positive, large coccobacillus; often in chains
Ana BAP
Medium, gray-white, opaque; sweet, fetid odor; colonies usually larger than most anaerobic cocci
Porphyromonas spp.
Gram-negative coccobacilli
Ana BAP
Dark brown to black; more mucoid than Prevotella spp.; except for P. gingivalis, fluoresces brick red
Prevotella disiens
Gram-negative rods; occur in pairs or short chains
Ana BAP
White, circular, entire, convex, translucent to opaque, smooth, shiny; nonhemolytic; fluoresces brick red
LKV
Black pigment
P. melaninogenica
Gram-negative coccobacilli
Ana BAP
Dark center with gray to light brown edges; circular, entire, convex, smooth, shiny; nonhemolytic; fluoresces brick red
LKV
Black pigment
Propionibacterium spp.
Gram-positive, pleomorphic, diphtheroid-like rod; club-shaped to palisade arrangements; called anaerobic diphtheroids
Ana BAP
Young colonies are small and white to gray-white and become larger and more yellowish tan with age; P. avidum is beta-hemolytic
Veillonella parvula
Gram-negative, tiny diplococci in clusters, pairs, and short chains; unusually large cocci, especially in clusters, suggests Megasphaera or Acidaminococcus spp.
Ana BAP
Small, almost transparent; grayish white; smooth, entire, opaque, butyrous; may show red fluorescence under UV light (360 nm)
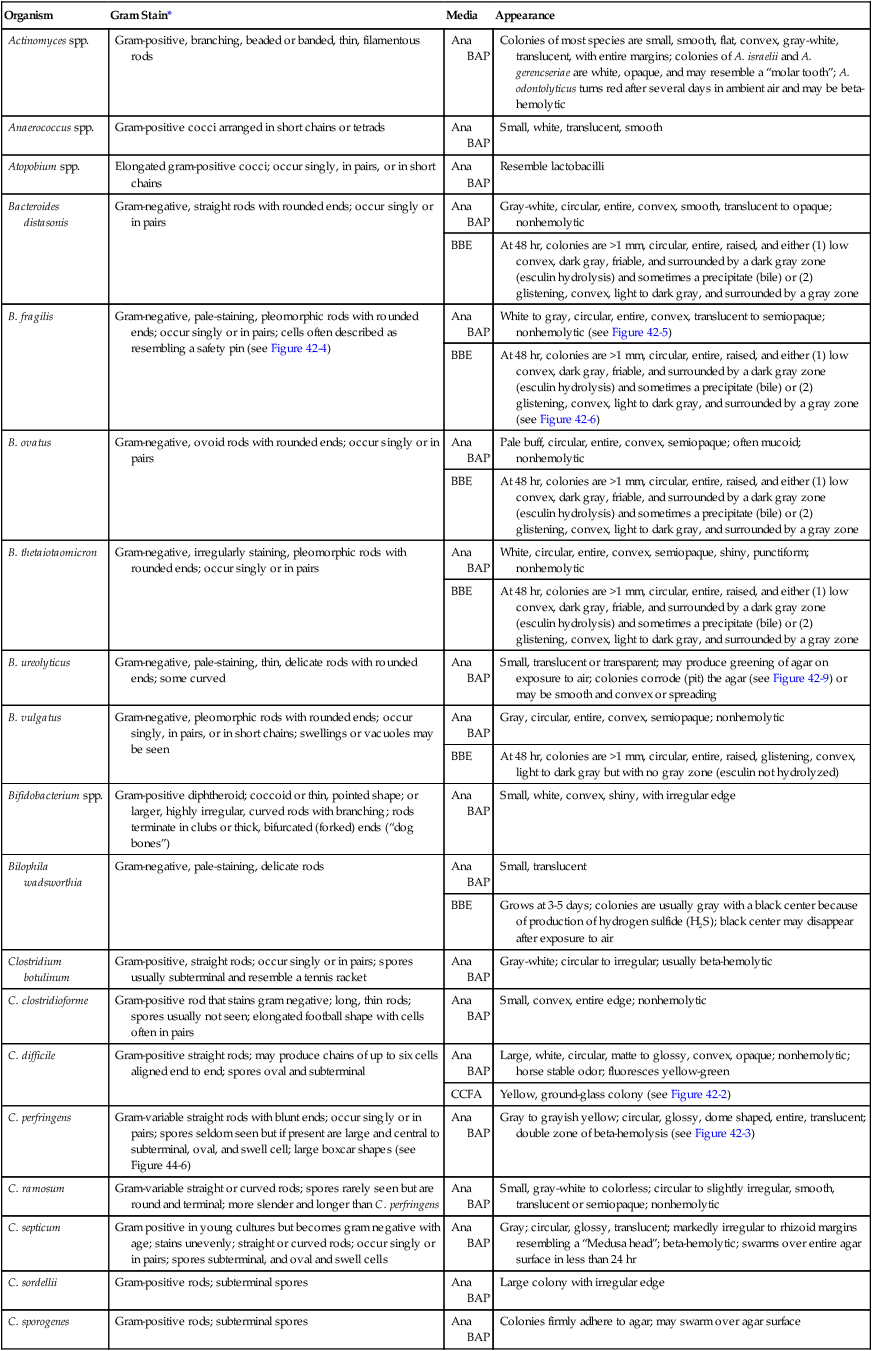
Specimen Processing
Anaerobe Jars or Pouches
Anaerobe Chamber
Anaerobic Media
Medium
Components/Comments
Primary Purpose
Anaerobic blood agar
May be prepared with Columbia, Schaedler, CDC, Brucella, or brain-heart infusion base supplemented with 5% sheep blood, 0.5% yeast extract, hemin, L-cystine, and vitamin K1
Nonselective medium for isolation of anaerobes and facultative anaerobes
Bacteroides bile esculin agar (BBE)
Trypticase soy agar base with ferric ammonium citrate and hemin; bile salts and gentamicin act as inhibitors
Selective and differential for Bacteroides fragilis group; good for presumptive identification
Laked kanamycin-vancomycin (LKV)
Brucella agar base with kanamycin (75 µg/ mL), vancomycin (7.5 µg/mL), vitamin K1 (10 µg/mL), and 5% laked blood
Selective for isolation of Prevotella and Bacteroides spp.
Anaerobic phenylethyl alcohol agar (PEA)
Nutrient agar base, 5% blood, phenylethyl alcohol
Selective for inhibition of enteric gram-negative rods and swarming by some clostridia
Egg yolk agar (EYA)
Egg yolk base
Nonselective for determination of lecithinase and lipase production by clostridia and fusobacteria
Cycloserine cefoxitin fructose agar (CCFA)
Egg yolk base with fructose, cycloserine (500 mg/L), and cefoxitin (16 mg/L); neutral red indicator
Selective for Clostridium difficile
Cooked meat (also called chopped meat) broth
Solid meat particles initiate growth of bacteria; reducing substances lower oxidation-reduction potential (Eh)
Nonselective for cultivation of anaerobic organisms; with addition of glucose, can be used for gas-liquid chromatography
Peptone–yeast extract–glucose broth (PYG)
Peptone base, yeast extract, glucose, cysteine (reducing agent), resazurin (oxygen tension indicator), salts
Nonselective for cultivation of anaerobic bacteria for gas-liquid chromatography
Thioglycollate broth
Pancreatic digest of casein, soy broth, and glucose to enrich growth of most bacteria. Thioglycollate and agar reduce Eh. May be supplemented with hemin and vitamin K1
Nonselective for cultivation of anaerobes, facultative anaerobes, and aerobes ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine