9.1 INTRODUCTION TO OUTCOMES OF INFECTION FOR THE HOST
In the previous few chapters we have looked at aspects of the virus replication cycle that culminate in the exit of infective progeny virions from an infected cell. When this is the outcome the infection is said to be productive. Virions may be released when the host cell lyses. Alternatively, the cell may survive releasing virions for a period, which may be short, as in the case of HIV infection, or long, as in the case of hepatitis B virus infection.
Some virus infections, however, are not productive, for a variety of reasons.
- An infection may become latent with the virus genome persisting, perhaps for the lifetime of the cell, and perhaps in the daughter cells if the cell divides.
- An infection may be abortive, in which case neither a latent infection nor a productive infection is established. One cause of abortive infection is a virus with a mutated genome; the virus is said to be defective. It is unable to undergo a complete replication cycle, unless the cell is also infected with a virus that can provide the missing function(s). A virus that is able to provide functions for a defective virus is known as a helper virus.
Some virus infections persist in their hosts for long periods, sometimes for life. In some cases persistent infections are productive, e.g. HIV; in other cases persistence may take the form of periods of latency alternating with periods of productive infection, e.g. many herpesviruses. Long-term infection with some viruses may lead to the development of cancer (Chapter 23).
Some phages, including the filamentous phages (Section 20.4.2), initiate persistent productive infections. Under appropriate conditions the host cells can survive for long periods releasing progeny virions.
The outcome of a virus infection from the point of view of the host may vary from harmless at one end of the scale, through deleterious effects of varying severity, to death at the other end of the scale. The outcome may hinge on complex interplay between a variety of host, viral, and environmental factors.
Over time the organisms that are hosts to viruses have evolved anti-viral defenses, but the viruses have not sat idly by. The relationship between a virus and its host usually involves an arms race and viruses have developed countermeasures against host defenses. Some of these countermeasures are indicated in boxes throughout this chapter.
In this chapter we concentrate mainly on virus infections in vertebrate hosts, and we start by considering some aspects of host immunity.
9.2 FACTORS AFFECTING OUTCOMES OF INFECTION
A major factor affecting the outcome of a virus infection is the efficiency of the host’s immune systems. In animals there are both innate and adaptive immune systems, each made up of a large number of molecules and cell types. There are links between the innate and adaptive immune systems; for example, aspects of the innate system stimulate the development of an adaptive immune response.
9.2.1 Innate immunity in vertebrates
Some aspects of innate immunity that viruses might encounter prior to infecting cells were introduced in Section 4.3.3. Further components of innate immunity that might be encountered after cells have become infected include complement, interferons, natural killer (NK) cells, APOBEC3 proteins, and tetherin.
9.2.1.a Complement
The complement system consists of cell surface receptors and soluble factors that can detect and respond to foreign invaders, including viruses. Complement can be activated by three pathways that result in modifications to complement proteins, with a number of possible outcomes for a virus. Complexes of modified complement proteins can insert themselves into membranes containing virus proteins, damaging enveloped virions and lysing infected cells. Some modified complement proteins can coat virions, which then attach to phagocytes (neutrophils and macrophages) via complement receptors on the phagocyte surface. Complement activation can also enhance adaptive immune responses.
9.2.1.b Interferons
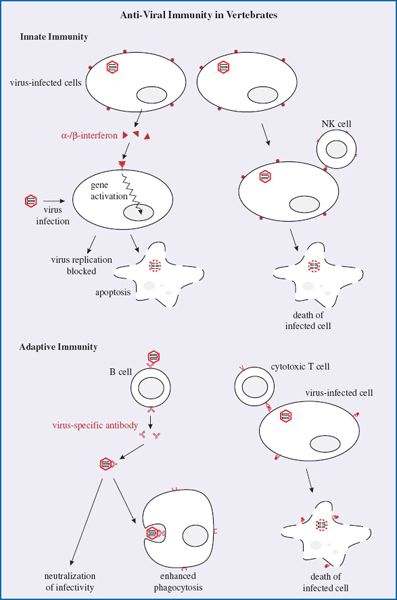
Interferons are proteins synthesized and secreted by cells in response to virus infection. The roles of interferons are to protect adjacent cells from infection and to activate T cell-mediated immunity (Section 9.2.2.b). Interferon synthesis is triggered by virus proteins and nucleic acids binding to pattern recognition receptors, of which there are various types located at the cell surface, within endosomes, and in the cytoplasm (Figure 9.1). One group of these receptors is known as Toll-like receptors; some of them are able to recognize virus nucleic acids as foreign.
Figure 9.1 Interferons. Virus components at the cell surface, within endosomes, and in the cytoplasm can bind to pattern recognition receptors, triggering the cell to synthesize interferons. Secreted interferons bind to receptors on other cells, activating a wide spectrum of genes. If these cells become infected the gene products may either block virus replication or cause the infected cells to die (apoptosis).
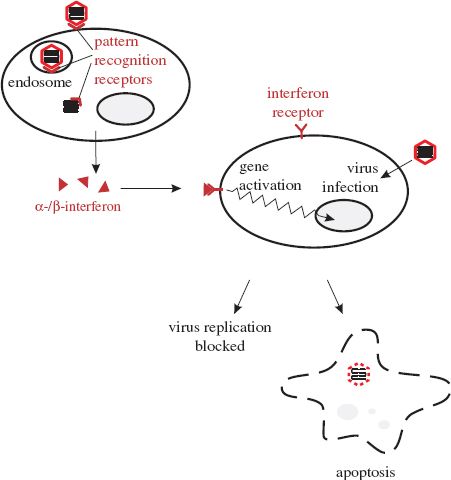
There are a number of types of interferon. Alpha- and beta- (α- and β-) interferons are produced by most cell types when they become infected with viruses. After secretion the interferons may bind to receptors on nearby cells (Figure 9.1), activating a large number of genes and triggering various anti-viral activities, including the following:
- activation of genes that encode antiviral proteins. One example of an interferon-induced antiviral protein is dsRNA-dependent protein kinase R. This enzyme is synthesized in an inactive form, but when it binds to dsRNA it can phosphorylate several substrates, resulting in either a block to virus replication or the death of the infected cell. Tetherin (Section 9.2.1.e) is another example of an interferon-induced antiviral protein;
- production of major histocompatibility (MHC) class I molecules and proteasome proteins; these molecules enhance the presentation on the infected cell surface of viral peptides to T cells;
- activation of NK cells (Section 9.2.1.c);
- induction of apoptosis (Section 9.2.4).
Another type of interferon, gamma- (γ-) interferon, is produced mainly by T cells and NK cells when triggered by certain molecules (e.g. interleukin-2) released during immune responses. γ-interferon has a number of effects, including stimulation of antigen presentation and activation of phagocytes and NK cells.
9.2.1.c Natural killer (NK) cells
NK cells are present throughout the body, but mainly in the blood. They are lymphocyte-like cells that can recognize changes in the surface molecules of virus-infected cells as a result of infection, though they do not recognize specific antigens, unlike B cells and T cells (Section 9.2.2). After recognizing virus-infected cells as target cells, NK cells are able to bind to them and then, as their name implies, kill them (Figure 9.2).
Figure 9.2 Activities of natural killer (NK) cells. After binding to a virus-infected cell, an NK cell releases γ-interferon and kills the infected cell.
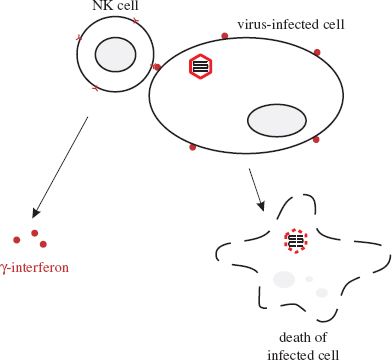
NK cells kill their target cells either by releasing perforins, which are proteins that are inserted into the plasma membrane of the virus-infected cell, or by inducing apoptosis (Section 9.2.4). Also, on binding to infected cells, NK cells release γ-interferon.
9.2.1.d APOBEC3 proteins
There are enzymes in the cells of humans and animals that can interfere with the replication of reverse transcribing viruses (retroviruses and hepadna-viruses). These enzymes can induce lethal mutations by deaminating deoxycytidine to deoxyuridine during reverse transcription. The enzymes delight in the name apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3 proteins; the abbreviation APOBEC3 proteins is easier to cope with!
Several of these proteins in human cells can interfere with the replication of HIV. Two of them (APOBEC3F and APOBEC3G) can be incorporated into HIV virions and taken into the next cell, where they can wreak havoc during reverse transcription.
9.2.1.e Tetherin
Tetherin is an interferon-induced protein that is expressed at the cell surface as dimers anchored in the plasma membrane. Tetherin can prevent the budding of enveloped viruses from the cell surface by forming a tether between the lipid membranes of the cell and the virion.
9.2.2 Adaptive immunity in vertebrates
An important outcome of virus infection in a vertebrate host is the development of a virus-specific immune response triggered by the virus antigens. Regions of antigens known as epitopes bind to specific receptors on lymphocytes, activating cascades of events that result in the adaptive immune response.
The key cells in specific immune responses are lymphocytes, of which there are two classes: B lymphocytes (B cells), which develop in the Bursa of Fabricius in birds and in the bone marrow in mammals, and T lymphocytes (T cells), which develop in the thymus. Each lymphocyte has epitope-specific receptors on its cell surface, making it specific for a particular epitope. Naive lymphocytes are those that have not encountered their specific epitopes; these cells have surface molecules and circulation patterns in the body distinct from lymphocytes that have previously encountered their epitopes.
9.2.2.a Antibodies
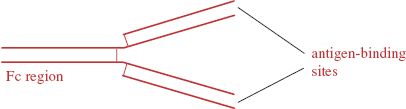
Antibodies are glycoproteins of a type known as immunoglobulins. The basic structure of an antibody molecule is shown in Figure 9.3. The molecule is constructed from two “heavy” and two “light” polypeptide chains and contains two antigen-binding sites and a region known as the Fc (Fragment crystallizable) region.
Figure 9.3 An antibody molecule. The molecule consists of four polypeptide chains held together by disulfide bonds. The chains are arranged to form two sites that can bind to a specific antigen. Fc: Fragment crystallizable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


