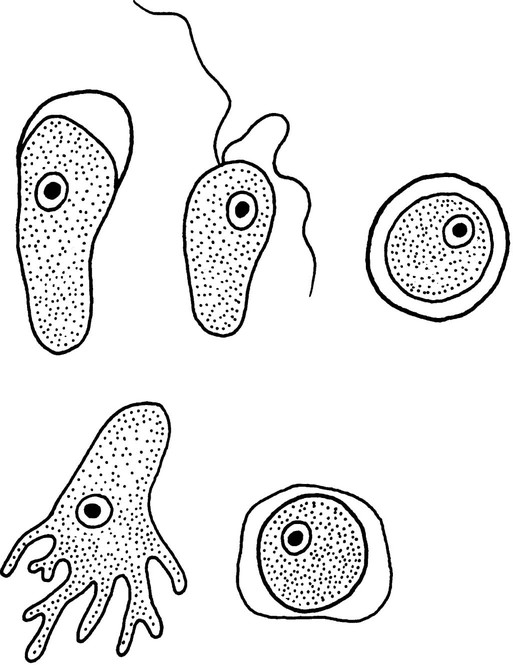
Chapter 50 1. Describe the distinguishing morphologic characteristics, clinical disease, basics of life cycle (source, stages of infectivity), and laboratory diagnosis for amebae, flagellates, and coccidia. 2. Compare and contrast the morphologic forms of the Naegleria trophozoites including specimens used for identification. 3. Compare and contrast Naegleria fowleri, Balamuthia mandrillaris, and Ancanthamoeba spp. including routes of transmission, specimens, risk factors, and disease presentation. 4. Compare and contrast the specimen requirements and morphologic characteristics of Pentatrichomonas hominis and Trichomonas vaginalis. 5. Identify the various morphologic forms of Toxoplasma gondii and correlate those with the clinical presentation of the infection (acute, chronic, and congenital). 6. Describe the various individual populations at risk for infection with Toxoplasma spp. and the disease symptoms and pathogenesis for each. 7. Define the following terms in relationship to the appropriate parasite discussed in this chapter: axostyle, bradyzoite, tachyzoite, ectocyst, mesocyst, endocyst, and oocyst. The trophozoites can occur in two forms: ameboid and flagellate (Table 50-1, Figure 50-1). The size ranges from 7 to 35 µm. The diameter of the rounded forms is usually 15 µm. There is a large, central karyosome and no peripheral nuclear chromatin. The cytoplasm is somewhat granular and contains vacuoles. The ameboid form organisms change to the transient, pear-shaped flagellate form when they are transferred from culture or teased from tissue into water and maintained at a temperature of 27° to 37° C. These flagellate forms do not divide, but when the flagella are lost, the ameboid forms resume reproduction. Cysts are generally round, measuring from 7 to 15 µm with a thick double wall. TABLE 50-1 Free-Living Amebae Causing Disease in Humans aAcanthamoeba in brain and Balamuthia in brain courtesy Dr. Govinda Visvesvara, Centers for Disease Control and Prevention. bIndirect immunofluorescence on tissue and PCR methods available from Centers for Disease Control and Prevention. Primary amebic meningoencephalitis (PAM) caused by N. fowleri is an acute, suppurative infection of the brain and meninges (Figure 50-2). With extremely rare exceptions, the disease is rapidly fatal in humans. The period between organism contact and onset of symptoms such as fever, headache, and rhinitis varies from a few days to 2 weeks. Early symptoms include vague upper respiratory tract distress, headache, lethargy, and occasionally olfactory problems. The acute phase includes sore throat; a stuffy, blocked, or discharging nose; and severe headache. Progressive symptoms include pyrexia, vomiting, and stiffness of the neck. Mental confusion and coma usually occur approximately 3 to 5 days before death, which is usually caused by cardiorespiratory arrest and pulmonary edema.
Other Protozoa
Naegleria Fowleri
General Characteristics
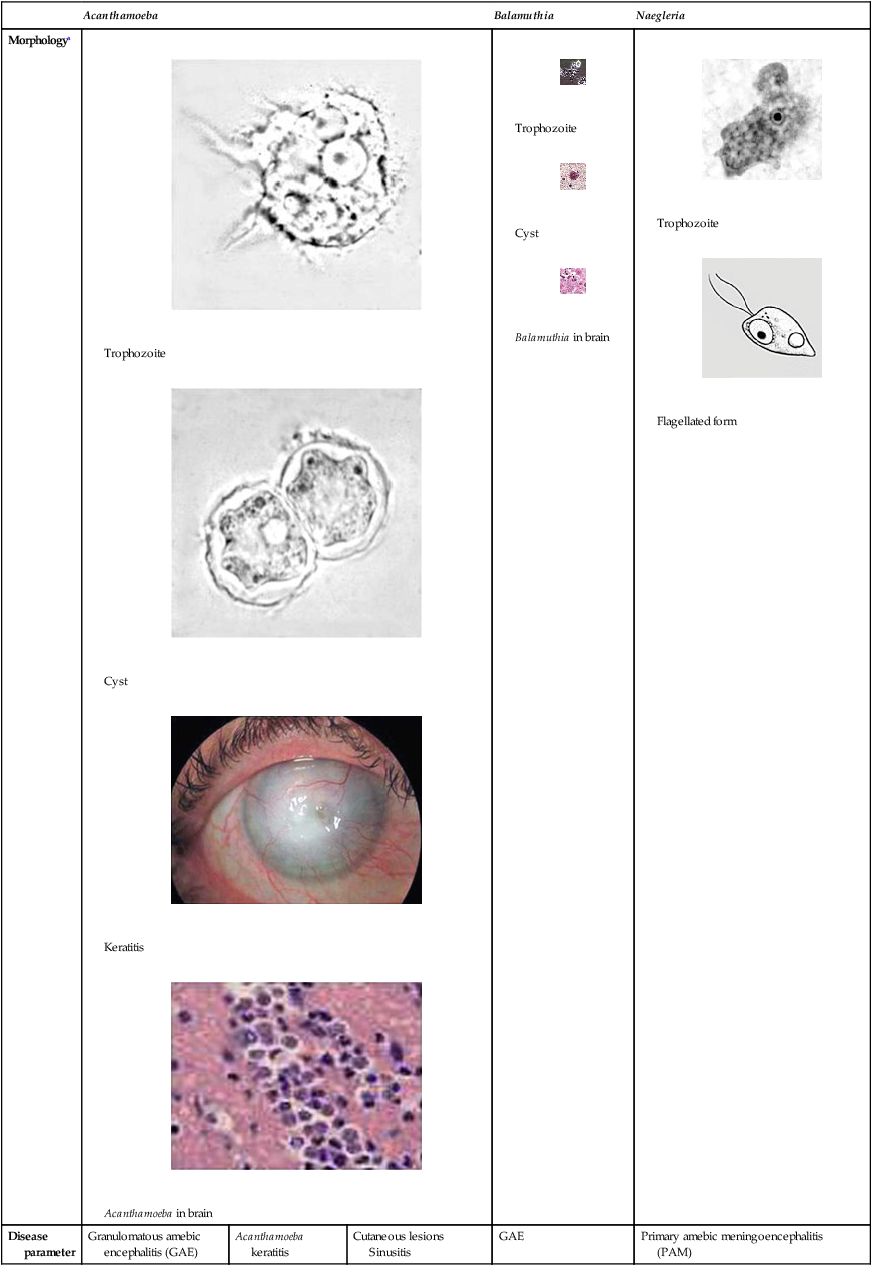
Acanthamoeba
Balamuthia
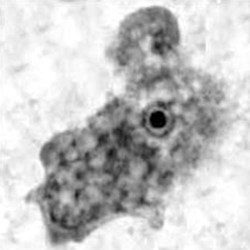
Naegleria
Morphologya
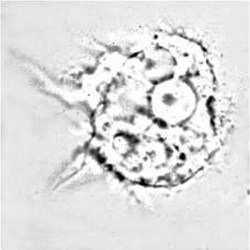
Trophozoite
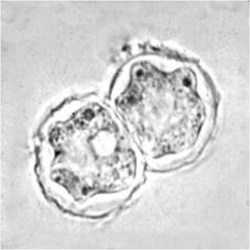
Cyst
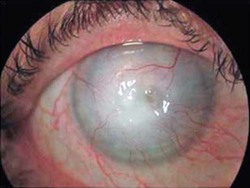
Keratitis
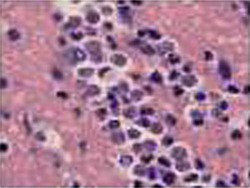
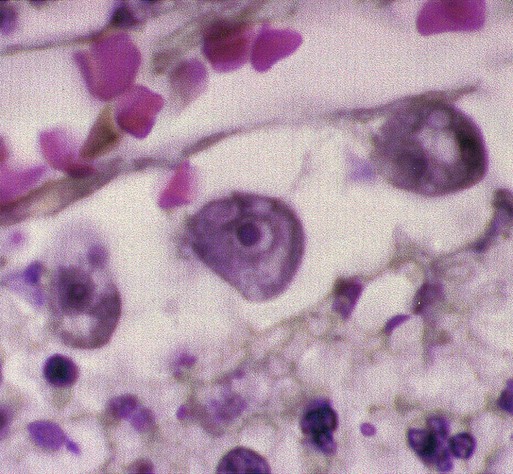
Acanthamoeba in brain
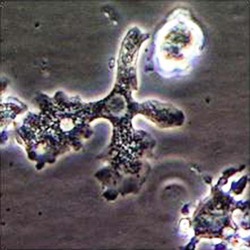
Trophozoite
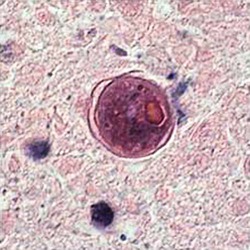
Cyst
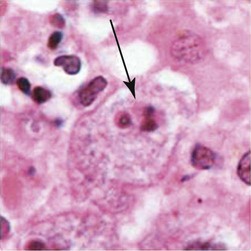
Balamuthia in brain
Trophozoite
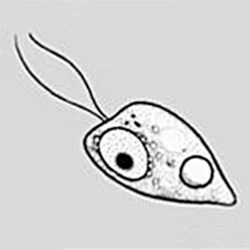
Flagellated form
Disease parameter
Granulomatous amebic encephalitis (GAE)
Acanthamoeba keratitis
Cutaneous lesions
Sinusitis
GAE
Primary amebic meningoencephalitis
(PAM)
General disease description
Chronic, protracted, slowly progressive CNS infection (may involve lungs); generally associated with individuals with underlying diseases
Painful, progressive, sight-threatening corneal disease; patients generally immunocompetent
Most common in patients with AIDS, with or without CNS involvement; those receiving immunosuppressive therapy for organ transplantation
Chronic, protracted, slowly progressive CNS infection (may involve lungs); generally associated with individuals with underlying diseases
Rare, but nearly always fatal infection; migration of amebae to brain through olfactory nerve; symptoms can mimic bacterial meningitis; death usually occurs 3-7 days after onset of symptoms; clinical suspicion based on history critical
Entry into body
Olfactory epithelium, respiratory tract, skin, sinuses
Corneal abrasion
Skin, sinuses, respiratory tract
Olfactory epithelium, skin, respiratory tract
Olfactory epithelium
Incubation period
Weeks to months
Days
Weeks to months
Weeks to months
Days
Clinical symptoms
Confusion, headache, stiff neck, irritability
Blurred vision, photophobia, inflammation, corneal ring, pain
Skin lesions, nodules, sinus lesions, sinusitis
Slurred speech, muscle weakness, headache, nausea, seizures
Headache, nausea, vomiting, confusion, fever, stiff neck, seizures, coma
Disease pathology
Focal necrosis, granulomas
Corneal ulceration
Granulomatous reaction in skin, inflammation
Multiple necrotic foci, inflammation, cerebral edema
Hemorrhagic necrosis
Diagnostic methods
Brain biopsy, CSF smear/wet prep, culture, indirect immunofluorescence on tissue,b PCRb
Corneal scrapings or biopsy, stain with calcofluor white, culture, confocal microscopy
Skin lesion biopsy, culture, indirect immunofluorescence of tissuea
Brain biopsy, culture on mammalian cells, indirect immunofluorescence of tissuea
Brain biopsy, CSF wet prep, culture, indirect immunofluorescence of tissue,a PCRa

Pathogenesis and Spectrum of Disease
Other Protozoa