Chapter 39 Osteoporosis is a common pathologic process of the skeleton in which bone strength is decreased. This predisposes the individual to increased risk of fracture. Bone strength is achieved through both bone density and factors such as bone architecture, cellular turnover, mineralization, and microfractures and other damage that all affect the quality of the bone. In osteoporosis, the normal coupling mechanism of bone breakdown and bone regrowth appears not to keep up with the constant microtrauma to trabecular bone, and either too little bone is formed or too much bone is removed. This ultimately results in a loss in bone amount and strength. Osteoporosis also may be produced by long-term glucocorticoid use, including use of inhaled steroids for treatment of chronic obstructive lung disease. Box 39-1 lists risk factors associated with osteoporosis. In periods of rapid remodeling (e.g., after menopause), bone may be at an increased risk for fracture because newly produced bone is less densely mineralized, the resorption sites are temporarily unfilled, and maturation and isomerization of collagen are impaired. The U.S. Preventive Services Task Force maintains that bone density testing is indicated for women and men with an increased risk for osteoporosis and routine screening to include all women 65 and older. If a woman is at least 5 years postmenopausal or has several risk factors, she should be strongly encouraged to have bone density testing and should be placed on therapy if the testing is indicative of osteoporosis or osteopenia. Box 39-2 lists indications for BMD testing. A T-score represents the number of SDs above or below the mean BMD for the young, healthy female population (i.e., women who are younger than 35 years of age). A T-score of −1 signifies a 10% to 12% loss of bone mass, compared with mean values for young, normal adults. According to recommendations of the WHO Task Force for Osteoporosis, osteoporosis is defined as a T-score −2.5 in women without a history of fragility fractures (Table 39-2). Treatment generally is indicated if the patient is two or more standard deviations below the normal premenopausal level. TABLE 39-2 WHO Criteria for Diagnosis of Osteoporosis for Postmenopausal Women and Men Aged 50 Years or Older ∗T-score indicates the number of standard deviations below the average peak bone mass in young adult females. • Qaseem A, Snow V, Shekelle P et al: Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians, Ann Intern Med 149(6):404-415, 2008. • U.S. Preventive Services Task Force (USPSTF) recommendations on screening for osteoporosis. 2011 update. Available at http://www.guidelines.gov/content.aspx?id=25316&search=osteoporosis. • Watts NB, Bilezikian JP, Camacho PM et al: American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis, Endocr Pract 16(3 Suppl):1-37, 2010. • Recommendation 1: Clinicians should offer pharmacologic treatment to men and women with known osteoporosis as well as to those who have experienced fragility fractures (strong recommendation; high-quality evidence). • Recommendation 2: For men and women who are at risk for the development of osteoporosis, clinicians should consider pharmacologic treatment (weak recommendation; moderate-quality evidence). Bisphosphonates are reasonable options to consider as first-line therapy, particularly for patients who have a high risk for hip fracture, because good-quality evidence shows that bisphosphonates lower the risk for vertebral, nonvertebral, and hip fractures. Although evidence from head-to-head trials is insufficient to show superiority of one bisphosphonate to another, alendronate and risedronate have been studied more than other bisphosphonates. • Recommendation 3: On the basis of evaluation of risk and benefits in individual patients, clinicians should select the most appropriate treatment options for osteoporosis in men and women (strong recommendation; moderate-quality evidence). • Recommendation 4: The ACP recommends that further research be performed to evaluate treatment options for osteoporosis in men and women. • The primary goal of osteoporosis management is to reduce fracture risk. Even when medication is initiated, nonpharmacologic efforts must be continued: activity to increase muscle and bone mass, strength, and flexibility, and reducing hazards such as loose rugs, defective stairs, poor lighting, and obstacles in the environment that present fall hazards. The American College of Physicians evidence-based guidelines suggest: • Pharmacotherapy for osteoporosis may affect different parts of the skeletal system differently; drugs that are effective for nonvertebral fractures are not necessarily effective for vertebral fractures. • Good evidence demonstrates that the bisphosphonates alendronate, etidronate, and risedronate lower the risk for vertebral, nonvertebral, and hip fractures and that ibandronate lowers the risk for vertebral fractures. Although no clear evidence suggests the optimal duration of treatment with bisphosphonates, trials to date have ranged from 3 months to 60 months in duration. • Estrogen is associated with a decreased risk for vertebral, nonvertebral, and hip fractures. Evidence for fracture risk reduction from combination therapy with vitamin D and calcium is stronger vs. calcium alone. For the vitamin D analogs 1,25(OH)D and 1(OH)D, evidence has shown a statistically significantly lower risk for vertebral fractures but mixed results for nonvertebral and hip fractures. • Gastrointestinal tract adverse events, including acid reflux, are increased with use of oral bisphosphonates. Although occurrence of mild upper gastrointestinal tract events with alendronate, ibandronate, risedronate, or zoledronic acid was not different from that seen with placebo in pooled analyses, there was an increased risk for mild gastrointestinal tract events in pooled analyses of 18 trials of etidronate vs. placebo. • In cardiovascular adverse events, contradictory evidence links zoledronic acid infusion to atrial fibrillation. However, raloxifene was associated with an increased pooled risk for pulmonary embolism and thromboembolic events, and estrogen was linked with an increased risk for cerebrovascular and thromboembolic events (Qaseem et al, 2008).
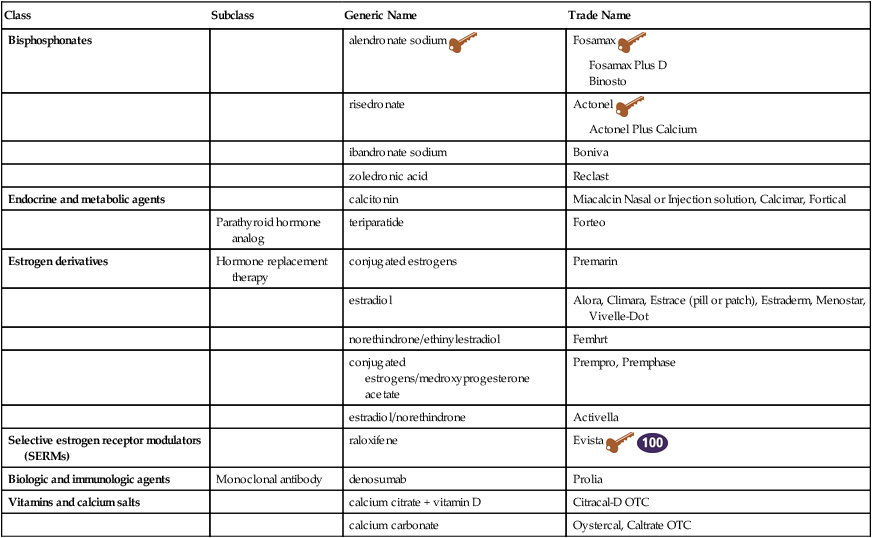
Osteoporosis Treatment
Class
Subclass
Generic Name
Trade Name
Bisphosphonates
alendronate sodium ![]()
Fosamax ![]()
Fosamax Plus D
Binosto
risedronate
Actonel ![]()
Actonel Plus Calcium
ibandronate sodium
Boniva
zoledronic acid
Reclast
Endocrine and metabolic agents
calcitonin
Miacalcin Nasal or Injection solution, Calcimar, Fortical
Parathyroid hormone analog
teriparatide
Forteo
Estrogen derivatives
Hormone replacement therapy
conjugated estrogens
Premarin
estradiol
Alora, Climara, Estrace (pill or patch), Estraderm, Menostar, Vivelle-Dot
norethindrone/ethinylestradiol
Femhrt
conjugated estrogens/medroxyprogesterone acetate
Prempro, Premphase
estradiol/norethindrone
Activella
Selective estrogen receptor modulators (SERMs)
raloxifene
Evista ![]()
![]()
Biologic and immunologic agents
Monoclonal antibody
denosumab
Prolia
Vitamins and calcium salts
calcium citrate + vitamin D
Citracal-D OTC
calcium carbonate
Oystercal, Caltrate OTC
Therapeutic Overview
Anatomy and Physiology
Pathophysiology
Assessment
T-Score∗
Classification
≥–1
Normal
≤ –1 to –2.5
Osteopenia (low bone mass)
≤ –2.5
Osteoporosis
≤ –2.5 + fracture
Severe osteoporosis
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations (from the American College of Physicians)
Cardinal Points of Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Osteoporosis Treatment
Only gold members can continue reading. Log In or Register to continue
