Osteoporosis and Other Metabolic Bone Diseases
KEY CONCEPTS
![]() Osteoporosis is a public health epidemic that affects all ages, genders, races, and ethnicities. Lifestyle behaviors, diseases, and medications should be reviewed to identify risk factors for developing osteoporosis and osteoporotic fractures. Healthcare practitioners should identify and resolve reversible risks. Patients with early onset or severe osteoporosis should be evaluated for secondary causes of bone loss.
Osteoporosis is a public health epidemic that affects all ages, genders, races, and ethnicities. Lifestyle behaviors, diseases, and medications should be reviewed to identify risk factors for developing osteoporosis and osteoporotic fractures. Healthcare practitioners should identify and resolve reversible risks. Patients with early onset or severe osteoporosis should be evaluated for secondary causes of bone loss.
![]() Bone physiology and pathophysiology are complex, involving many different cell lines, pathways, and biofeedback systems. As these processes become more delineated, opportunities for additional drug targets exist creating new classes of investigational agents.
Bone physiology and pathophysiology are complex, involving many different cell lines, pathways, and biofeedback systems. As these processes become more delineated, opportunities for additional drug targets exist creating new classes of investigational agents.
![]() An adult’s 10-year probability of developing an osteoporotic fracture can be estimated with the World Health Organization fracture risk assessment (FRAX) tool. Central bone densitometry can determine bone mass, predict fracture risk, and influence patient and provider treatment decisions. Portable equipment can be used for osteoporosis screening in the community to determine the need for further testing.
An adult’s 10-year probability of developing an osteoporotic fracture can be estimated with the World Health Organization fracture risk assessment (FRAX) tool. Central bone densitometry can determine bone mass, predict fracture risk, and influence patient and provider treatment decisions. Portable equipment can be used for osteoporosis screening in the community to determine the need for further testing.
![]() All people throughout their life spans should incorporate a bone-healthy lifestyle, which emphasizes regular exercise, nutritious diet, tobacco avoidance, minimal alcohol use, and fall prevention to prevent and treat osteoporosis.
All people throughout their life spans should incorporate a bone-healthy lifestyle, which emphasizes regular exercise, nutritious diet, tobacco avoidance, minimal alcohol use, and fall prevention to prevent and treat osteoporosis.
![]() Treatment should be considered for men or women older than age 50 years who have a hip or vertebral fracture, T-score ≤ –2.5 at the femoral neck or spine or those who have low bone mass (T-score between –1.0 and –2.5) at the femoral neck or spine and a 10-year probability of major osteoporotic fracture of ≥20% or hip fracture of ≥3% based on FRAX.
Treatment should be considered for men or women older than age 50 years who have a hip or vertebral fracture, T-score ≤ –2.5 at the femoral neck or spine or those who have low bone mass (T-score between –1.0 and –2.5) at the femoral neck or spine and a 10-year probability of major osteoporotic fracture of ≥20% or hip fracture of ≥3% based on FRAX.
![]() The recommended dietary allowance for calcium in American adults is 1,000 to 1,200 mg of elemental calcium daily with diet as the preferred source. Supplements are only added when diet is insufficient.
The recommended dietary allowance for calcium in American adults is 1,000 to 1,200 mg of elemental calcium daily with diet as the preferred source. Supplements are only added when diet is insufficient.
![]() The recommended dietary allowance for vitamin D for American adults is 600 units and for older adults 800 units daily, with some organizations and guidelines recommending higher doses of at least 800 to 1000 units daily. The daily target is achieved through sun exposure, fortified foods, and supplements. Vitamin D insufficiency, usually defined as 25-hydroxy vitamin D concentrations of <30 ng/mL (<75 nmol/L), is common in Americans.
The recommended dietary allowance for vitamin D for American adults is 600 units and for older adults 800 units daily, with some organizations and guidelines recommending higher doses of at least 800 to 1000 units daily. The daily target is achieved through sun exposure, fortified foods, and supplements. Vitamin D insufficiency, usually defined as 25-hydroxy vitamin D concentrations of <30 ng/mL (<75 nmol/L), is common in Americans.
![]() Alendronate, risedronate, zoledronic acid, and denosumab decrease vertebral, hip, and nonvertebral fractures and are considered first-line osteoporosis treatments. Ibandronate and raloxifene are alternatives, and calcitonin is a last-line agent. Teriparatide is reserved for severe osteoporosis or for those intolerant to other medications.
Alendronate, risedronate, zoledronic acid, and denosumab decrease vertebral, hip, and nonvertebral fractures and are considered first-line osteoporosis treatments. Ibandronate and raloxifene are alternatives, and calcitonin is a last-line agent. Teriparatide is reserved for severe osteoporosis or for those intolerant to other medications.
![]() Adherence with osteoporosis medications is frequently suboptimal, and poor adherence is associated with less fracture prevention. Healthcare providers have an important role in prevention and treatment of osteoporosis by assessing medication administration and adherence and by providing additional medication and disease education.
Adherence with osteoporosis medications is frequently suboptimal, and poor adherence is associated with less fracture prevention. Healthcare providers have an important role in prevention and treatment of osteoporosis by assessing medication administration and adherence and by providing additional medication and disease education.
![]() Patients taking long-term oral glucocorticoids and certain chemotherapeutic agents need to be identified and started on a bone-healthy lifestyle and usually should receive a bisphosphonate, denosumab, or teriparatide therapy to prevent or treat drug-induced osteoporosis.
Patients taking long-term oral glucocorticoids and certain chemotherapeutic agents need to be identified and started on a bone-healthy lifestyle and usually should receive a bisphosphonate, denosumab, or teriparatide therapy to prevent or treat drug-induced osteoporosis.
![]() Osteoporosis is a bone disorder characterized by low bone density, impaired bone architecture, and compromised bone strength that predisposes a person to increased fracture risk.1 Osteoporosis is a major public health threat, especially with 55% of the Americans 50 years of age and older expected to have this disease.2 In the United States, 8 million women and 2 million men are estimated to have osteoporosis. The at-risk population is also large, with low bone density (osteopenia) estimates of 34 million Americans2 and 37% to 50% of white women.1
Osteoporosis is a bone disorder characterized by low bone density, impaired bone architecture, and compromised bone strength that predisposes a person to increased fracture risk.1 Osteoporosis is a major public health threat, especially with 55% of the Americans 50 years of age and older expected to have this disease.2 In the United States, 8 million women and 2 million men are estimated to have osteoporosis. The at-risk population is also large, with low bone density (osteopenia) estimates of 34 million Americans2 and 37% to 50% of white women.1
Attention to bone health is needed in people of all ages. The development of osteoporosis and osteoporotic fractures is multifactorial, beginning at birth with genetics and then throughout life as a result of health behaviors that influence bone growth and maintenance, skeletal factors that lead to compromised bone strength, and nonskeletal factors that lead to falls (Fig. 73–1). Therefore all healthcare providers should educate everyone about prevention, especially providing encouragement to practice a bone-healthy lifestyle, monitor bone health in patients at risk, and provide optimal treatment for patients with osteoporosis.
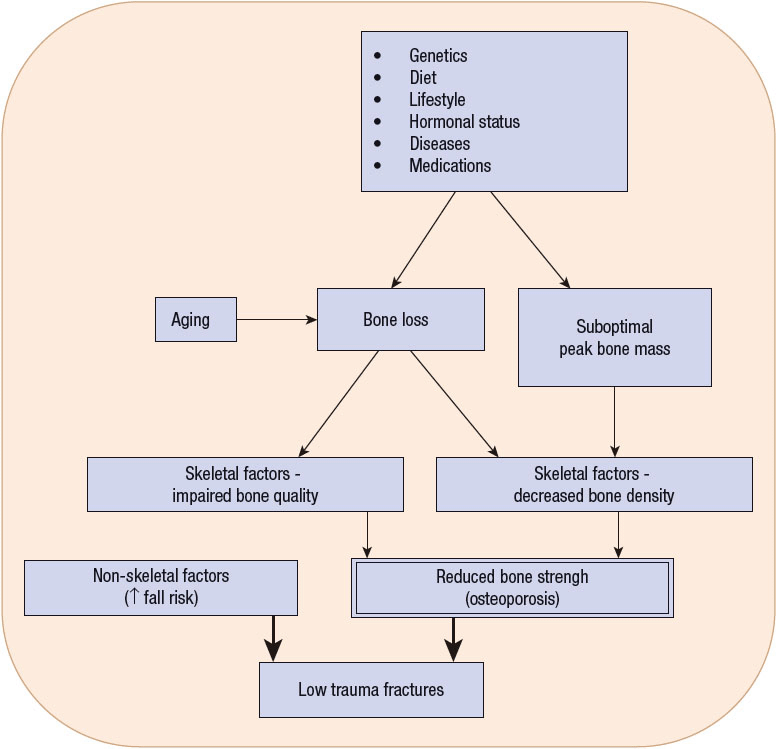
FIGURE 73-1 Etiology of osteoporosis and osteoporotic fractures.
EPIDEMIOLOGY
![]() Low bone density, osteoporosis, and osteoporotic fractures are very common and affect all ethnic groups. Low bone density is estimated to occur in 52% of white and Asian, 49% of Hispanic, and 35% of black women age 50 and older.2 Osteoporosis affects 20% of white and Asian, 10% of Hispanic, and 5% of black women age 50 and older. Disease prevalence greatly increases with age; from 4% in women 50 to 59 years of age to 44% to 52% in women 80 years of age and older.1 White and Hispanic women have the highest fragility fracture (those occurring after falls from no more than a standing height and with minimal or no trauma) rate followed by Native American, African American, and Asian women when the data are adjusted for weight, bone mineral density (BMD), and other factors. Approximately 20% to 27% of men aged 50 years and older have low bone density rising to 49% in men 80 years and older.3 Osteoporosis prevalence in non-Hispanic white men is 4% to 5%, non-Hispanic black men is 3%, and Hispanic men is 2%. Osteoporosis prevalence rises to 17% in men 80 years and older. Although osteoporosis is a common finding in older adults with fractures, up to 50% of fragility fractures occur in patients with normal or low bone mass.1
Low bone density, osteoporosis, and osteoporotic fractures are very common and affect all ethnic groups. Low bone density is estimated to occur in 52% of white and Asian, 49% of Hispanic, and 35% of black women age 50 and older.2 Osteoporosis affects 20% of white and Asian, 10% of Hispanic, and 5% of black women age 50 and older. Disease prevalence greatly increases with age; from 4% in women 50 to 59 years of age to 44% to 52% in women 80 years of age and older.1 White and Hispanic women have the highest fragility fracture (those occurring after falls from no more than a standing height and with minimal or no trauma) rate followed by Native American, African American, and Asian women when the data are adjusted for weight, bone mineral density (BMD), and other factors. Approximately 20% to 27% of men aged 50 years and older have low bone density rising to 49% in men 80 years and older.3 Osteoporosis prevalence in non-Hispanic white men is 4% to 5%, non-Hispanic black men is 3%, and Hispanic men is 2%. Osteoporosis prevalence rises to 17% in men 80 years and older. Although osteoporosis is a common finding in older adults with fractures, up to 50% of fragility fractures occur in patients with normal or low bone mass.1
Fragility wrist and vertebral fractures are common throughout adulthood, and hip fractures are more common in older adults. Fracture incidence has been estimated at 2 million (71% in women, 29% in men) in 2005, with an estimated total medical cost of $17 billion.4 Fractures in women accounted for 75% of the costs and in older adults 87% of the costs. Hip fractures represented 72% of these costs. Forecasting predicts 3 million fractures at a cost of $25 billion in 2025. The incidences of hip fracture and associated mortality are decreasing for both genders,5 with the hypothesis related to better efforts at osteoporosis prevention (e.g., bone-healthy lifestyle) and use of bisphosphonates. In a woman’s lifetime, she has a 17% likelihood of a hip fracture, 15.6% likelihood of a vertebral fracture, and 16% likelihood of a forearm fracture.1 In a man’s lifetime, osteoporotic fracture risk is 13% to 30%. However, rates in the United States remain higher than those in other countries, and comorbidities are increasing,5 suggesting a need for continued focus on bone health.
ETIOLOGY
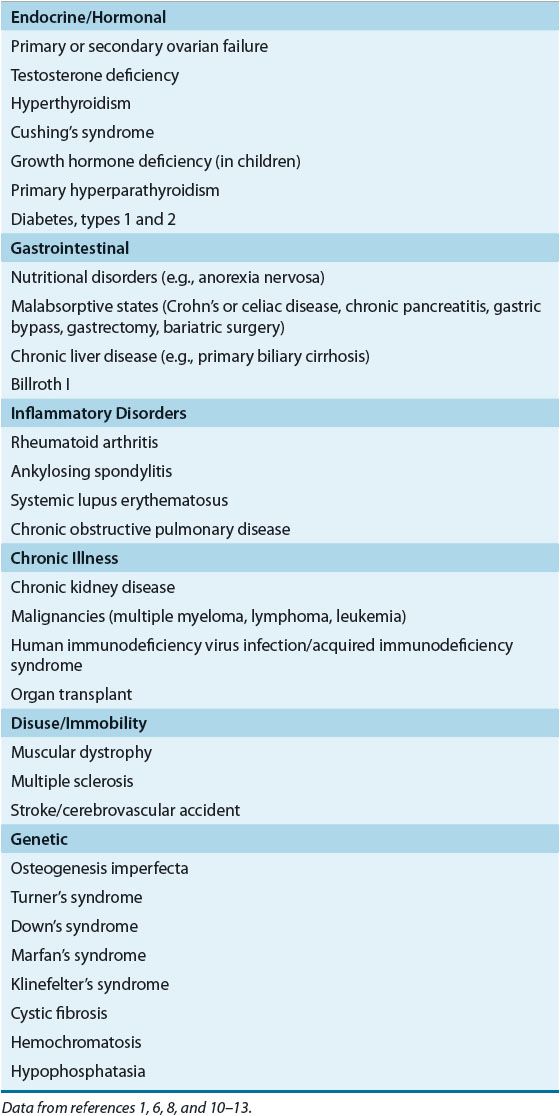
![]() Figure 73–1 depicts a model describing the etiology of osteoporosis and fractures. Table 73–11,6–9 lists risk factors for osteoporosis, and Tables 73–21,6,8,10–13 and 73-310,14,15 list secondary causes of this condition.
Figure 73–1 depicts a model describing the etiology of osteoporosis and fractures. Table 73–11,6–9 lists risk factors for osteoporosis, and Tables 73–21,6,8,10–13 and 73-310,14,15 list secondary causes of this condition.
TABLE 73-1 Risk Factors for Osteoporosis and Osteoporotic Fractures
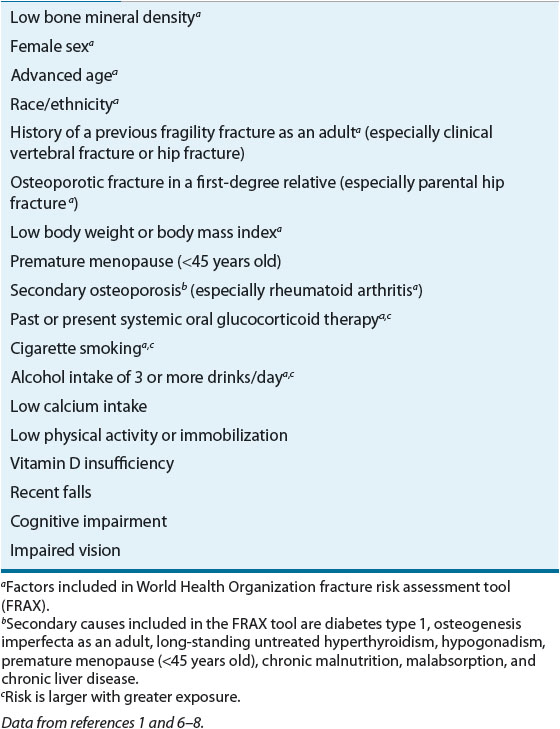
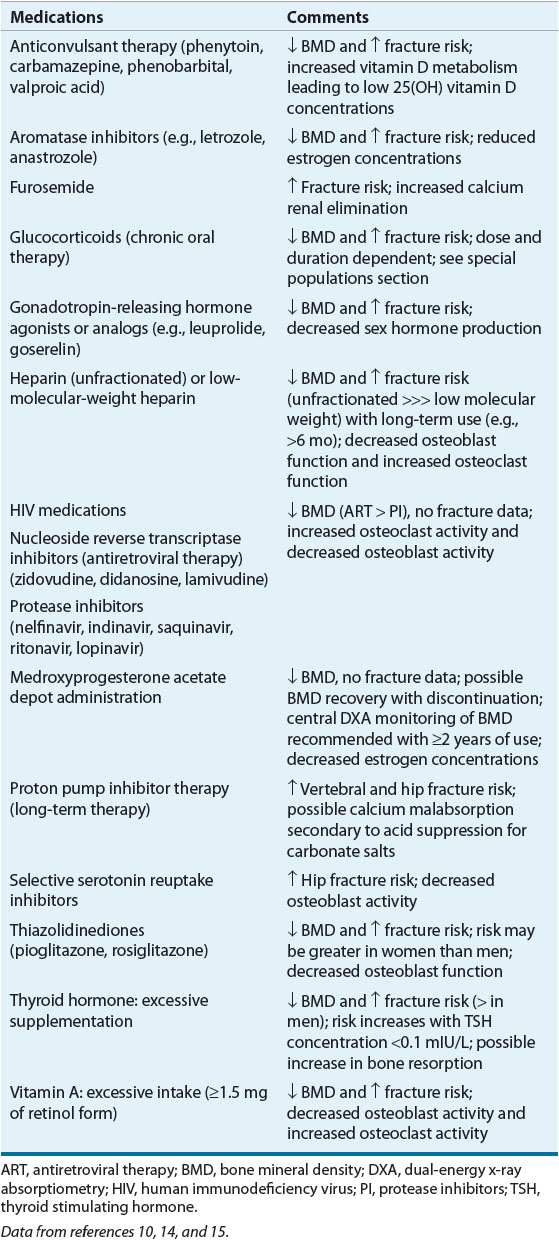
TABLE 73-3 Selected Medications Associated with Increased Bone Loss and/or Fracture Risk
Low Bone Density
BMD is a major predictor of fracture risk. Every standard deviation decrease in BMD in women represents a 10% to 12% decrease in bone mass and a 1.5- to 2.6-fold increase in fracture risk.2 Low BMD can occur as a result of failure to reach a normal peak bone mass or bone loss. Bone loss occurs when bone resorption exceeds bone formation, usually from high bone turnover; when the number or depth of bone resorption sites greatly exceeds the rate and ability of osteoblasts to form new bone. Women and men begin to lose a small amount of bone mass starting in the third to fourth decade of life as a consequence of a slight reduction in bone formation.16 During perimenopause and menopause, bone loss occurs predominantly due to increases in bone resorption secondary to estrogen deficiency. Older adults steadily lose bone mass as a consequence of an accelerated rate of bone remodeling combined with reduced bone formation.
The major risk factors (see Tables 73–1, 73-2, and 73-3) influencing bone loss are hormonal status, exercise, aging, nutrition, lifestyle, disease states, medications, and some genetic influences. Nonhormonal risk factors are similar between women and men.
Impaired Bone Quality
In addition to BMD, the strength of bone is highly affected by the quality of the bone’s composition and its structure.16 For example, besides decreasing bone mass, accelerated bone turnover can also impair bone quality and the structural integrity of bone by increasing the quantity of immature bone that is not yet adequately mineralized. In men, the bone loss that results from thinning of trabeculae with aging is less damaging to the quality and strength of bone structure than bone loss in women where damage to trabecular crosslinks is seen. Bone quality assessment is important because changes in bone quality affect bone strength much more than bone mass changes. Future osteoporosis diagnostic testing will assess both bone quality and density.
Falls
Although up to 50% of vertebral fractures can occur spontaneously with minimal to no trauma, most wrist fractures and greater than 90% of hip fractures result from a fall from standing height or less.2 One-third to one-half of all older adults fall each year, and 50% fall more than once. Up to 5% of all falls will result in a fracture. According to 2006 statistics, 2.1 million older adults were treated in the emergency department and 600,000 hospitalized for fall-related injuries, incurring costs of about $20 billion.17 Close to 17,000 older adults died in 2006 due to a fall-related injury.18
PATHOPHYSIOLOGY
Bone Physiology
The skeleton has two types of bone.19 Cortical bone makes up the majority of the skeleton (80%) and is found mostly in the long bones (e.g., forearm and hip). Trabecular bone is found mostly in the vertebrae and ends of long bones. This bone type is 10 times more metabolically active compared with cortical bone due to a much higher bone turnover rate because of its large surface area and honeycomb-like shape.
Bone is made of collagen and mineral components.19 The collagen component gives bone its flexibility and energy-absorbing capability. The mineral component gives bone its stiffness and strength. The correct balance of these substances is needed for bone to adequately accommodate stress and strain and resist fractures. Imbalances can impair bone quality and lead to reduced bone strength.
Bone strength reflects the integration of bone mass and bone quality (composition and microarchitecture).19 Bone mass increases rapidly throughout childhood and adolescence. Peak bone mass is attained by age 18 to 21 years.6,20 Peak bone mass is highly dependent on genetic factors that account for approximately 60% to 80% of the variability.8 The remaining 20% to 40% is influenced by modifiable factors such as nutritional intake (e.g., calcium, vitamin D, and protein), exercise, adverse lifestyle practices (e.g., smoking), hormonal status, and certain diseases and medications. Optimizing peak bone mass is important for preventing osteoporosis. The higher the peak bone mass, the more bone one can lose before being at an increased fracture risk. As the microarchitecture of bone deteriorates, the bone strength greatly decreases. Women loose more bone structure than men.6
![]() Bone remodeling is a dynamic process that occurs continuously throughout life Figure 73–2A–C.19,21–26 One to two million tiny sections of bone are in the process of remodeling at any given time. Many cytokines, growth factors, and hormones influence each remodeling step. The complete physiology of bone remodeling is not fully known but appears to begin with signals from lining cells or osteocytes (bone communication cells) that are triggered by stress, microfractures, biofeedback systems responsive to cytokines and growth factors, and potentially certain diseases and medications (see Fig. 73–2B, step 1). A major stimulus for hematopoietic stem cell (monocyte–macrophage lineage) differentiation to become mature osteoclasts (bone-resorbing cells) is the receptor activator of nuclear factor kappa B ligand (RANKL), which is emitted from the osteoblast (bone-forming cells) in step 2. Interleukin 1 and 6, colony-stimulating factor, parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), 1,25(OH) vitamin D, tissue growth factor-β (TGF-β), prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) stimulate RANKL release whereas estrogen, calcitonin, and estrogen agonists antagonists (EAAs) inhibit RANKL release. The RANKL then binds to its receptor, receptor activator of nuclear factor-κB (RANK), on the surface of osteoclast precursors initiating differentiation. The RANKL also stimulates mature osteoclast activation and bone adherence via αvβ3 integrins to resorb bone (step 3). This step is influenced by TGF-β, insulin-like growth factor-1 and 2 (IGF), platelet derived growth factor (PDGF), bone morphometric protein (BMP), and fibroblast growth factor (FGF). After bone attachment, the osteoclasts secrete proteinases, such as cathepsin K, collagenase, gelatinase, tartrate-resistant acid phosphate (TRAP), and matrix metalloproteases, and hydrogen ions to dissolve the mineralized bone. The hydrogen ion production is under src kinase control, which needs to be bound to other compounds such as Cbl, Fak, and phosphatidylinositol 3′-kinase (Pl3K).
Bone remodeling is a dynamic process that occurs continuously throughout life Figure 73–2A–C.19,21–26 One to two million tiny sections of bone are in the process of remodeling at any given time. Many cytokines, growth factors, and hormones influence each remodeling step. The complete physiology of bone remodeling is not fully known but appears to begin with signals from lining cells or osteocytes (bone communication cells) that are triggered by stress, microfractures, biofeedback systems responsive to cytokines and growth factors, and potentially certain diseases and medications (see Fig. 73–2B, step 1). A major stimulus for hematopoietic stem cell (monocyte–macrophage lineage) differentiation to become mature osteoclasts (bone-resorbing cells) is the receptor activator of nuclear factor kappa B ligand (RANKL), which is emitted from the osteoblast (bone-forming cells) in step 2. Interleukin 1 and 6, colony-stimulating factor, parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), 1,25(OH) vitamin D, tissue growth factor-β (TGF-β), prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) stimulate RANKL release whereas estrogen, calcitonin, and estrogen agonists antagonists (EAAs) inhibit RANKL release. The RANKL then binds to its receptor, receptor activator of nuclear factor-κB (RANK), on the surface of osteoclast precursors initiating differentiation. The RANKL also stimulates mature osteoclast activation and bone adherence via αvβ3 integrins to resorb bone (step 3). This step is influenced by TGF-β, insulin-like growth factor-1 and 2 (IGF), platelet derived growth factor (PDGF), bone morphometric protein (BMP), and fibroblast growth factor (FGF). After bone attachment, the osteoclasts secrete proteinases, such as cathepsin K, collagenase, gelatinase, tartrate-resistant acid phosphate (TRAP), and matrix metalloproteases, and hydrogen ions to dissolve the mineralized bone. The hydrogen ion production is under src kinase control, which needs to be bound to other compounds such as Cbl, Fak, and phosphatidylinositol 3′-kinase (Pl3K).
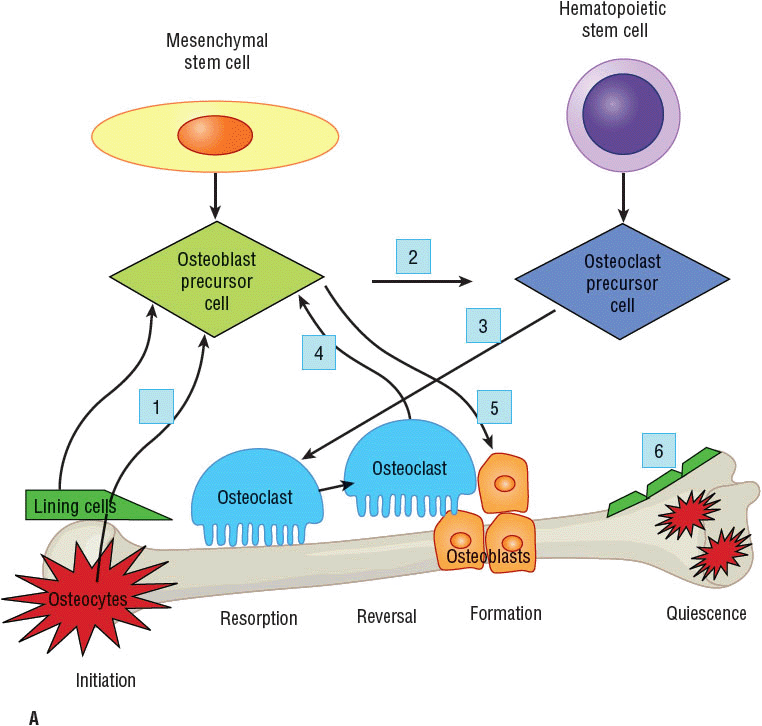
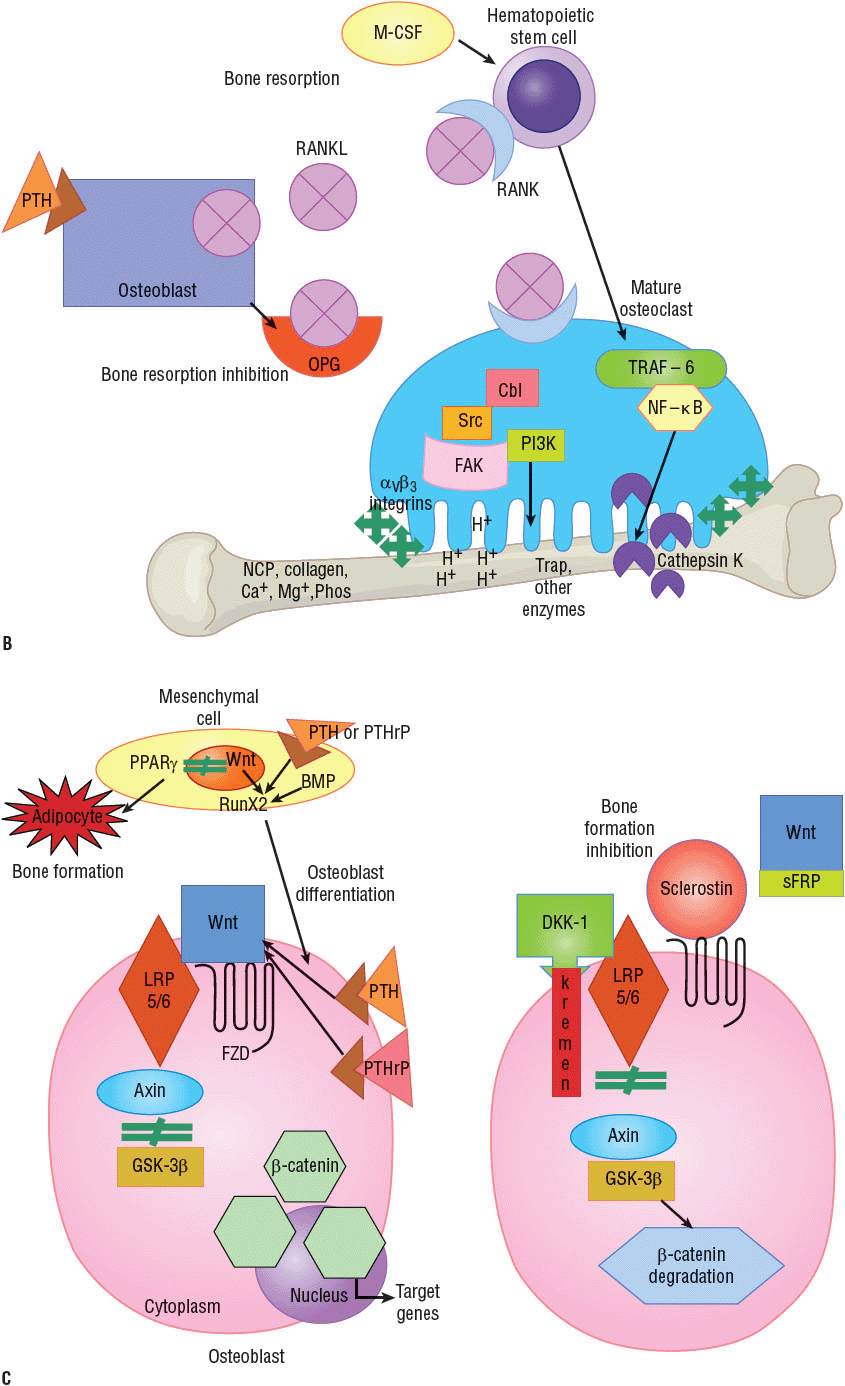
FIGURE 73-2 Bone remodeling cycle.19,21–26 A. Overview of remodeling process: step 1, initiation; steps 2 and 3, resorption; step 4, reversal; step 5, formation; and step 6, quiescence.
B. Molecular level detail of major pathways during bone resorption steps 2 and 3, which also showcase drug targets for approved and investigational agents. (Ca+, calcium ion; Cbl, a ubiquitin ligase; FAK, focal adhesion kinase; H+, hydrogen ion; M-CSF, macrophage colony-stimulating factor; Mg+, magnesium ion; NCP, noncollagenous protein; NF-κB, nuclear factor kappa B; OPG, osteoprotegerin; Pl3K, phosphatidylinositol 3′-kinase; Phos, phosphorus; PTH, parathyroid hormone; RANK, receptor activator of nuclear factor-κB; RANKL, receptor activator of nuclear factor-κB ligand; src, tyrosine-protein kinase; TRAF-6, tumor necrosis factor receptor associated factor 6; Trap, tartrate-resistant acid phosphate.) C. Molecular level detail of major pathways during bone formation steps 4 and 5, which also showcase drug targets for approved and investigational agents. (BMP, bone morphogenetic protein; DKK-1, Dickkoff-1; FZD, frizzled element; GSK-3β, glycogen synthase kinase-3β; LRP5/6, lipoprotein receptor-related protein 5 or 6; PPAR-γ, peroxisome proliferator-activated receptor-γ; PTH, parathyroid hormone; PTHrP, parathyroid hormone-related protein; runX2, runt-related transcription factor; sFRP, secreted frizzled related protein; Wnt, wingless tail ligand.)
After bone is resorbed and a cavity is created, osteoclasts produce ephrinB2 that adheres to ephB4 receptors on osteoblasts and along with additional cytokines and growth factors elicit osteoblast differentiation from mesenchymal stem cells, maturation and activity (step 4). PTH and PTHrP also directly increase osteoblast differentiation and activity. Osteoblast differentiation can be inhibited by leptin and peroxisome proliferator-activated receptor–γ (PPAR-γ), which direct mesenchymal cell maturation to adipocytes instead of osteoblasts. Mature osteoblasts produce osteoprotegerin (OPG) that binds to RANKL, thereby stopping bone resorption.
Bone formation occurs over two phases (see Fig. 73–2C).22–25 First the wingless tail ligand (Wnt) binds to low-density lipoprotein receptor–related protein 5 or 6 (LRP5/6) and a frizzled element (FZD). Wnt function is also influenced by PTH and PTHrP, which fit into the same receptor. Next LRP5/6 binds to axin, which then cannot bind to glycogen synthase kinase-3β (GSK-3β), thus preventing degradation of β-catenin (step 5). β-catenin then enters the nucleus and signals target genes to create proteins to fill the resorption cavity with osteoid. Growth hormone and IGF-1 also increase bone collagen production. The second phase is the mineralization of bone with calcium, magnesium and phosphorus to give it strength.
Once the cavity is mineralized, bone formation can be stopped by at least three processes. Sclerostin, predominantly secreted from osteocytes, and/or Dickkoff-1 (DKK-1) can bind to LRP5/6 or secreted frizzled related proteins (sFRP) can bind to Wnt to prevent Wnt signaling. Axin can then bind to GSK-3β, which then can cause β-catenin degradation, osteoblast apoptosis, and the end of osteoblastic activity (step 6). The mature osteoblasts can become lining cells or osteocytes. Recent discoveries have found osteocytes to be very biologically active producing OPG to stop resorption and sclerostin and DKK-1, to stop bone formation, with ongoing research to determine the triggers for this cell.25 Quiescence is the phase when bone is at rest until another remodeling cycle is initiated.
Estrogen has many positive effects on the bone remodeling process, with most of its actions helping to maintain a normal bone resorption rate.21 Estrogen suppresses the proliferation and differentiation of osteoclasts and increases osteoclast apoptosis. Estrogen decreases the production of several cytokines that are potent stimulators of osteoclasts, including interleukins (ILs) 1 and 6, TNF-α, and macrophage colony-stimulating factor (M-CSF), and increases TGF-α, which increases osteoclast apoptosis. Estrogen also decreases the production of RANKL, increases the production of OPG and TGF-α, which reduce osteoclastogenesis. Osteoclast apoptosis increases by activating Fas/FasL signaling.
Testosterone’s role in bone health is becoming more apparent with recent identification of some direct effects on bone resorption and osteoblasts.21 Most of testosterone’s bone effects relate to its metabolism to estradiol and the above bone effects of estrogens. Testosterone can also increase OPG production, which will inhibit bone resorption. Increased osteoblast proliferation and differentiation are direct effects. These effects might be from increasing TGF-β, TGF mRNA, FGF, and IGF-2, and decreasing IL-6.
Vitamin D, Parathyroid Hormone, and Calcium
Vitamin D and PTH work together to maintain calcium homeostasis. The most abundant source of vitamin D is the endogenous production from skin exposure to ultraviolet B light. The sun’s ultraviolet B light converts 7-dehydrocholesterol in the skin to cholecalciferol (vitamin D3). Dietary vitamin D sources and supplements include cholecalciferol and ergocalciferol (vitamin D2). Subsequent conversion of cholecalciferol and ergocalciferol to 25-hydroxyvitamin D [25(OH) vitamin D] (calcidiol) occurs in the liver, and then PTH stimulates conversion of 25(OH) vitamin D via 25(OH) vitamin D-1α-hydroxylase (cytochrome P450 [CYP]27B1) to its final active form, 1α,25-dihydroxyvitamin D (calcitriol), in the kidney. Calcitriol binds to the intestinal vitamin D receptor (VDR) and then increases calcium-binding proteins calmodulin and calbindin. As a result, calcium and phosphorous intestinal absorption is increased. The feedback system is completed with CYP27B1 activity inhibited by adequate calcium and phosphorus, and FGF-23. Vitamin D receptors and CYP27B1 are also found in many tissues, such as bone, intestine, brain, breast, colon, heart, stomach, pancreas, lymphocytes, skin, and gonads. Vitamin D is increasingly recognized as contributing to many nonbone benefits, and those may relate to the presence of these conversion capabilities and receptors throughout the body.
Calcium absorption under normal conditions is approximately 30% to 40%, decreasing to 10% to 15% with low vitamin D concentrations. Calcium absorption is thus lower in winter and is reported higher in obesity. Calcium absorption is predominantly an active rate-limited process in the duodenum and jejunum, which is controlled by many hormones such as 1,25 dihydroxyvitamin D and estrogen and TRPV6, which is under genomic control and responsive to dietary calcium intake. A calcium transporter (calmodulin or calbindin) is required to bring calcium from the gut into the tissue wall and then across the enterocyte. Calcium is extruded into the circulation via Ca2+ adenosine triphosphatase (ATPase) and the sodium/calcium exchanger (NCX), high energy steps. Another absorption method is paracellular passive diffusion throughout the intestine, which counts for less than 15% of absorbed calcium, is not rate limited, and possibility is sensitive to 1,25 dihydroxyvitamin D as well. Solvent drag plays a minor role in calcium absorption.
When the calcium-sensing receptor (CaSR) on parathyroid cells senses low serum calcium, PTH production increases. PTH then increases calcitriol production and calcium reabsorption by the kidney. Calcium absorption increases as 25(OH) vitamin D concentrations increase until 29 to 32 ng/mL (72 to 80 nmol/L) when the effect plateaus; this observation provides the rationale for the cutoff point for vitamin D sufficiency being around 30 ng/mL (75 nmol/L). Sometimes the increased fractional calcium absorption is insufficient to maintain normal serum calcium, and thus bone resorption is needed for correction. Together, PTH and calcitriol increase RANKL and osteoclast activity, thereby releasing calcium from bone to restore calcium homeostasis.
Osteoporosis pathophysiology depends on sex, age, diet, genetics, and presence of secondary causes.
Postmenopausal Osteoporosis
Estrogen deficiency causes significant bone density loss and compromises bone architecture. Estrogen deficiency increases proliferation, differentiation, and activation of new osteoclasts and prolongs survival of mature osteoclasts.21 Interleukins, prostaglandin E2, TNF-α, and interferon γ also increase resulting in more RANKL and less OPG. Loss of estrogen also increases calcium excretion and decreases calcium gut absorption through decreases in TRPV6 activity and 1,25 dihydroxy vitamin D binding proteins.27 The results of this deficiency can also be seen in other settings such as anorexia nervosa and during lactation, and with medications such as prolonged depot medroxyprogesterone acetate implants, aromatase inhibitors and gonadotropin releasing hormone agonists.14,21
During menopause, trabecular bone is most susceptible, leading to vertebral and wrist fractures. Accelerated bone loss begins during perimenopause and continues for 3 to 4 years after menopause from bone resorption exceeding formation.1,20 During this time annual bone loss can be as high as 2%, with total BMD loss due to menopause approximately 6% to 7%.1,20 The number of remodeling sites increases, and resorption pits are deeper and inadequately filled by normal osteoblastic function.
Male Osteoporosis
Male bone also decreases with decreased testosterone.3,6,21,28,29 Men do not undergo a period of accelerated bone resorption similar to menopause, but slowly decreasing testosterone concentrations with aging and or conditions or medications causing hypogonadism cause bone loss. Although the major effect of low testosterone is the loss of metabolism to estradiol, some direct positive bone effects are loss as well. Men are at a lower risk for developing osteoporosis and osteoporotic fractures because of larger bone size, greater peak bone mass, increase in bone width with aging, fewer falls, and shorter life expectancy. However, mortality rate after a fracture is greater for men than women.
The etiology of male osteoporosis tends to be multifactorial (see Tables 73–2 and 73-3). Most common risk factors for men are smoking, low body weight, weight loss, age, long-term glucocorticoid use, androgen deprivation therapy, and low testosterone concentrations.3
Age-Related Osteoporosis
Age-related osteoporosis occurs in older adults because of accelerated bone turnover rate and reduced osteoblast bone formation.30 These bone changes result from hormone,31 calcium,27 and vitamin D27 deficiencies and/or changes in their absorption and metabolism, decreased production or function of cytokines or other bone biochemicals, increases in redox status and free radical formation, increases in adipocytes, telomere shortening, and less exercise. Approximately 0.5% BMD is loss each year after age 60 years.8 Fracture risk for a given BMD value increases with aging. Hip fracture risk rises dramatically in older adults as a consequence of the cumulative loss of cortical and trabecular bone and an increased risk for falls. Aging is associated with muscle changes as well, resulting in weakness, balance instability, and greater likelihood of falls.31
Secondary Causes of Osteoporosis
![]() Osteoporosis often has secondary causes (see Tables 73–2 and 73-3).1,6,8,10–15 Symptoms, initial screening laboratory test results, medication profile review, and or an elevated Z-score from a dual-energy absorptiometry (DXA) test can suggest a secondary cause could be present, warranting more comprehensive workups.
Osteoporosis often has secondary causes (see Tables 73–2 and 73-3).1,6,8,10–15 Symptoms, initial screening laboratory test results, medication profile review, and or an elevated Z-score from a dual-energy absorptiometry (DXA) test can suggest a secondary cause could be present, warranting more comprehensive workups.
CLINICAL PRESENTATION
Osteoporosis is diagnosed by BMD measurement or presence of a fragility fracture. Many vertebral fractures are asymptomatic, with patients sometimes attributing mild lower back pain to “old age.” Some fractures present with moderate-to-severe back pain that radiates down the leg after a new vertebral fracture. The pain usually subsides significantly after 2 to 4 weeks; however, residual chronic lower back pain can persist. Multiple vertebral fractures decrease height and sometimes curve the spine (kyphosis or lordosis) with or without significant back pain. Patients who have experienced a nonvertebral fracture frequently present with severe pain, swelling, and reduced function and mobility at the fracture site.
CLINICAL PRESENTATION
Consequences of Osteoporosis
A fragility fracture is defined as one that occurs as a result of a fall from standing height or less or with minimal to no trauma, sometimes referred to as atraumatic fracture. Fractures of the vertebrae, hip, forearm, or humerus are considered major osteoporotic fractures. Fractures of the face, skull, fingers, and toes are typically not considered osteoporosis-related. Osteoporotic fractures can lead to increased morbidity and mortality and decreased quality of life. Depression is common because of fear, pain, loss of self-esteem from physical deformity, and loss of independence and mobility.
Symptomatic vertebral fractures can cause significant pain, physical deformity, and adverse health consequences. Patients with severe kyphosis can experience respiratory problems as a result of compression of the thoracic region and gastrointestinal complications, such as poor nutrition, from intraabdominal compression. Women and men who suffer a symptomatic vertebral fracture have a lower survival rate compared with those without a fracture history.7
Wrist fractures occur more commonly in younger postmenopausal women and are frequently a result of a fall on an outstretched hand. Negative outcomes include prolonged pain and weakness, and decreased instrumental, (advanced) activities of daily living (such as cooking and shopping).
Hip fractures are associated with the greatest increase in morbidity and mortality. In 2007, hip fractures resulted in approximately 281,000 hospital admissions in those age 65 and older.32 After a hip fracture, only 50% of patients regain their ability to perform basic activities of daily living, while 20% become nonambulatory.33 Of patients age 50 and over, almost one-quarter die within 1 year either from complications of the hip fracture or other comorbid disease processes.2 Men have a twofold higher 1-year mortality rate after hip fracture than women.
Once a low-trauma fracture has occurred, the risk for subsequent fractures goes up exponentially. Peri- or post-menopausal women with a history of fracture have double the risk of a subsequent fracture compared to women without a fracture history.1 In older women with two or more vertebral fractures, the risk of a new fracture is 12-fold higher, than for subjects who did not have baseline fractures.
PATIENT ASSESSMENT
Bone pain, postural changes (i.e., kyphosis), and loss of height are simple useful physical examination findings. A height loss greater than 1.5 inches (3.8 cm) from the tallest mature height is considered significant and warrants further investigation.34,35 Height should be routinely measured using a wall-mounted stadiometer. Proper technique is essential; height is frequently measured incorrectly.36 A spine radiograph can be obtained to confirm the presence of vertebral fractures. Low bone density or osteopenia reported on routine radiographs is a sign of significant bone loss and requires further evaluation for osteoporosis. In addition to physical examination and laboratory studies (see Clinical Presentation),1,6,8,10,20,37,38 patients can be assessed with risk factor assessments, osteoporosis questionnaires, peripheral and central DXA or ultrasonography, and bone turnover biomarkers.5
Risk Factor Assessment
The aim of an initial osteoporosis risk assessment (see Table 73–1) is to identify those patients who are at highest risk for low bone density and who would benefit from further evaluation. Many risk factors for osteoporosis have been identified and are similar for both sexes. The majority of risk factors are predictors of either low BMD (e.g., female sex, ethnicity) or an increased fracture and fall risk (e.g., cognitive impairment, previous falls). The most important risk factors are those associated with fracture risk independent of BMD and fall risk. These major risk factors, in combination with BMD, are used to determine which patients are at greatest risk for fracturing and would benefit most from pharmacologic intervention.
![]() A fracture prediction model used for treatment risk stratification was developed for the World Health Organization (WHO).9,39 The WHO model for the United States uses the following risk factors: age, race/ethnicity, sex, previous fragility fracture, parent history of hip fracture, body mass index, glucocorticoid use (current or past use for 3 or more months of prednisolone 5 mg daily or equivalent doses of other glucocorticoids), current smoking, alcohol use of three or more drinks per day, rheumatoid arthritis, and select secondary causes with femoral neck BMD data optional to predict an individual’s percent probability of fracturing in the next 10 years. The WHO fracture risk assessment (FRAX) tool can be used to help predict fracture risk in patients who do not have access to DXA. The FRAX model should not be used to predict fracture risk in patients already on therapy for osteoporosis although under investigation. Some risk factors for fracture are not accommodated in the FRAX model.9 For example, falls are a risk factor for fracture, but at this time researchers are unable to quantify the risk to add it to the model. Therefore, clinicians should use the FRAX model but also continue to assess all risk factors in an osteoporosis risk assessment.
A fracture prediction model used for treatment risk stratification was developed for the World Health Organization (WHO).9,39 The WHO model for the United States uses the following risk factors: age, race/ethnicity, sex, previous fragility fracture, parent history of hip fracture, body mass index, glucocorticoid use (current or past use for 3 or more months of prednisolone 5 mg daily or equivalent doses of other glucocorticoids), current smoking, alcohol use of three or more drinks per day, rheumatoid arthritis, and select secondary causes with femoral neck BMD data optional to predict an individual’s percent probability of fracturing in the next 10 years. The WHO fracture risk assessment (FRAX) tool can be used to help predict fracture risk in patients who do not have access to DXA. The FRAX model should not be used to predict fracture risk in patients already on therapy for osteoporosis although under investigation. Some risk factors for fracture are not accommodated in the FRAX model.9 For example, falls are a risk factor for fracture, but at this time researchers are unable to quantify the risk to add it to the model. Therefore, clinicians should use the FRAX model but also continue to assess all risk factors in an osteoporosis risk assessment.
Screening Using Peripheral Bone Mineral Density Devices
Peripheral bone density devices that use DXA (pDXA) or quantitative ultrasonography are helpful as screening tools to determine which patients require further evaluation with central DXA or for decision making if central DXA testing is not available.34,35 pDXA of the forearm, heel, and finger uses a low amount of radiation and requires personnel with special training. Heel quantitative ultrasonography uses sound waves without radiation or need for special training. Heel ultrasonography has better fracture predictive value than pDXA. The specific peripheral T-score threshold for referral is not universally defined and varies by device. These tests should not be used for diagnosis or for monitoring response to therapy.
Because peripheral devices are considerably less expensive than central DXA, easy to use, portable, fast (<5 minutes), and can predict general fracture risk, they are very popular for screening patients at health fairs, community pharmacies, and clinics. No guidelines specifically address screenings, but it is reasonable to limit use to postmenopausal women and men 65 years and older for whom results are predictive of future fracture risk.7,35 Healthy premenopausal women generally should not be screened. Patients already identified as being at high risk for osteoporosis based on risk factors, fragility fracture, or secondary causes for osteoporosis should not be screened but rather referred to a physician for central DXA testing.
Central Dual-Energy X-ray Absorptiometry
![]() BMD measurements at the hip or spine (or radius if these bones cannot be scanned) can be used to assess fracture risk, establish the diagnosis and severity of osteoporosis, and sometimes confirm osteoporosis as causative for low-trauma fractures.1,6–8,35 Central DXA is considered the gold standard for measuring BMD because of its high precision, short scan times, low radiation dose (comparable to the average daily dose from natural background), and stable calibration. Measurement of lumbar spine, proximal femur, and total hip BMD is recommended with the lowest BMD value used for diagnosis. Newer methods, such as micromagnetic resonance imaging, are undergoing investigation to provide measurements of bone quality in addition to bone density.
BMD measurements at the hip or spine (or radius if these bones cannot be scanned) can be used to assess fracture risk, establish the diagnosis and severity of osteoporosis, and sometimes confirm osteoporosis as causative for low-trauma fractures.1,6–8,35 Central DXA is considered the gold standard for measuring BMD because of its high precision, short scan times, low radiation dose (comparable to the average daily dose from natural background), and stable calibration. Measurement of lumbar spine, proximal femur, and total hip BMD is recommended with the lowest BMD value used for diagnosis. Newer methods, such as micromagnetic resonance imaging, are undergoing investigation to provide measurements of bone quality in addition to bone density.
Several consensus guidelines or position statements are consistent in recommending central BMD testing for all women aged 65 years or older, men aged 70 years or older, postmenopausal women younger than 65 years of age and men 50 to 69 years old with risk factors for fracture, and patients with an identified secondary cause for bone loss. The United States Preventive Services Task Force agrees with respect to women 65 years and older, but for women between 50 to 65 years old, the task force recommends getting a DXA only for those women with a FRAX major osteoporotic fracture score ≥9.3%.40 They feel data are inadequate to make recommendations for men. Patients with a fragility fracture do not need a DXA for an osteoporosis diagnosis, but the results are helpful for determining disease severity and as a baseline for monitoring therapy effects. The DXA results can help patients make decisions about the need for lifestyle changes and prescription osteoporosis medications. In the absence of a suspected or known secondary cause for osteoporosis or a history of a low-trauma fracture, central BMD testing is not recommended for children, premenopausal women, or men younger than 50 years of age.
A central DXA BMD report provides the actual bone density value, T-score, and Z-score.34,35 The actual bone density value (g/cm2) is most useful for serial monitoring of therapy response, which is typically performed 1 to 2 years after medication initiation. The T-score is used for diagnosis and is a comparison of the patient’s measured BMD to the mean BMD of a healthy, young (20- to 29-year-old), sex-matched white reference population; no adjustments for race or ethnicity. The T-score is the number of standard deviations from the mean of the reference population. The Z-score is similar but compares the patient’s BMD to the mean BMD for a healthy sex- and age-matched population. Patient-reported race or ethnicity should be used for the Z-score if available. A Z-score value of ≤ –2.0 is sometimes helpful in determining whether a secondary cause for osteoporosis is present and is used for diagnosis in children, premenopausal women, and men younger than 50 years of age. Followup monitoring for patients identified with normal or low bone density has not been clearly defined, but one study has suggested a recall period of 15 years for those with T-scores > –1.49, 5 years for T-scores between –1.50 and –1.99, and 1 year for T-score between –2.00 and –2.49.41
Using the spine DXA image, an assessment of morphometric vertebral fractures, the vertebral fracture assessment (VFA), can be calculated.1,35 Each vertebra is assessed for compression (wedge, biconcave, and crush) and described as normal or mild (20% to 25%), moderate (25% to 40%), or severe (>40%) compression. This result becomes important for treatment decisions in patients with low bone mass. VFA is recommended in women >70 years old and in men >80 years old.6,7,35 They further recommend testing in younger postmenopausal women and men with low bone mass (T-score –1.5 or below) or specific risk factors, such as loss of height.
Laboratory Tests
Laboratory testing (see Clinical Presentation) is used to identify secondary causes of bone loss.1,6,10,20 If a preliminary investigation indicates a possible secondary cause, additional testing related to that specific secondary cause will be conducted.
Serum 25(OH) vitamin D is the best indicator of total body vitamin D status.1,42,43 The cutpoints for normal, insufficiency, and deficiency are controversial.43,44 Osteomalacia or severe vitamin D deficiency, which is discussed later in this chapter, can occur at concentrations less than 10 ng/mL (25 nmol/L).
Clinical Controversy…
Because vitamin D assays are fairly expensive and large inter-laboratory assay variability exists, routine vitamin D screening cannot be recommended at this time. However, a 25(OH) vitamin D concentration should be considered in anyone at higher risk for low vitamin D (e.g., older adults, patients who are obese or who have minimal sun exposure, insufficient vitamin D intake, dark-pigmented skin, or certain medical conditions especially liver and kidney disease, or are on medications known to affect vitamin D metabolism), and patients with low bone density, history of a low-trauma fracture or frequent falls, or history of unexplained muscle weakness and/or bone pain.42
Bone Turnover Markers
Increased concentrations of bone resorption markers (≥2 standard deviations above the premenopausal range) have been shown in some studies to predict fracture risk; however, results have been inconsistent.38 Bone turnover markers are commonly used in clinical trials and have documented responses to therapy and predictive of fracture prevention in some but not all studies.
Clinical utility is less since analytical and patient variability (within patient variability 26% to 43%) limit interpretation and accuracy. Circadian variability, seasonal variations, food intake, and recent exercise can all affect results. Although not diagnostic, these tests might be helpful in identifying accelerated bone turnover and increased fracture risk; however, most of these data are derived from osteoporosis studies in women. Bone turnover markers could be used to monitor therapy effects. Response to therapy can be measured as early as 2 to 3 months, but their utility is less for zoledronic acid and denosumab.1,6,37
Urine and serum bone turnover markers are either enzymes or proteins produced during bone formation or breakdown.1,6,37 Bone-specific alkaline phosphatase, osteocalcin, and procollagen type 1 propeptides (P1NP) are examples of bone formation markers. Hydroxypyridinium crosslinks of collagen pyridinoline and deoxypyridinoline, C-terminal crosslinking telopeptide of type 1 collagen (CTX), and N-terminal crosslinking telopeptide of type 1 collagen (NTX) are examples of bone resorption markers. CTX and P1NP, newer tests that are more accurate, are becoming the preferred tests. For serum markers, fasting morning samples should be obtained with repeat tests done at the same facility with the same assay.
DIAGNOSIS OF OSTEOPOROSIS
The diagnosis of osteoporosis is based on a low-trauma fracture or central hip and/or spine DXA using WHO T-score thresholds. Low bone mass (which is the preferred term) or osteopenia is a T-score between –1 and –2.5, and osteoporosis is a T-score at or below –2.5.1,6–8,34,35 Although these definitions are based on data from postmenopausal white women, they are also applied to perimenopausal women, men age 50 years and older, and adults from different races and ethnicities. The diagnosis of osteoporosis in children, premenopausal women, and men younger than 50 years of age should be based on a Z-score at or below –2.0 in combination with other risk factors or fracture.34,35 Children are not given a diagnosis of osteoporosis; “low BMD for chronological age” is the preferred term.34
PREVENTION AND TREATMENT
Osteoporosis
The foundation for osteoporosis prevention and treatment is a bone healthy lifestyle beginning at birth and continuing throughout life. Supplements and medications are used when lifestyle habits are suboptimal and/or osteoporosis has developed.
Desired Outcomes
General Approach to Prevention and Treatment
![]()
![]() A bone-healthy lifestyle should begin at birth and continue throughout life. Insuring adequate intakes of calcium and vitamin D along with other bone-healthy lifestyle practices are the first steps in prevention and treatment. Recent guidelines and position statements recommend considering prescription therapy in any postmenopausal woman or man age 50 years and older presenting with one of the following scenarios: a hip or vertebral fracture; T-score of –2.5 or lower at the femoral neck, total hip or spine; or low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) with a 10-year probability of hip fracture of 3% or more, or a 10-year probability of any major osteoporosis-related fracture of 20% or more.1,6–8 Figure 73–3 provides an osteoporosis management algorithm for postmenopausal women and men 50 years and older that incorporates both nonpharmacologic and pharmacologic approaches.
A bone-healthy lifestyle should begin at birth and continue throughout life. Insuring adequate intakes of calcium and vitamin D along with other bone-healthy lifestyle practices are the first steps in prevention and treatment. Recent guidelines and position statements recommend considering prescription therapy in any postmenopausal woman or man age 50 years and older presenting with one of the following scenarios: a hip or vertebral fracture; T-score of –2.5 or lower at the femoral neck, total hip or spine; or low bone mass (T-score between –1.0 and –2.5 at the femoral neck or spine) with a 10-year probability of hip fracture of 3% or more, or a 10-year probability of any major osteoporosis-related fracture of 20% or more.1,6–8 Figure 73–3 provides an osteoporosis management algorithm for postmenopausal women and men 50 years and older that incorporates both nonpharmacologic and pharmacologic approaches.
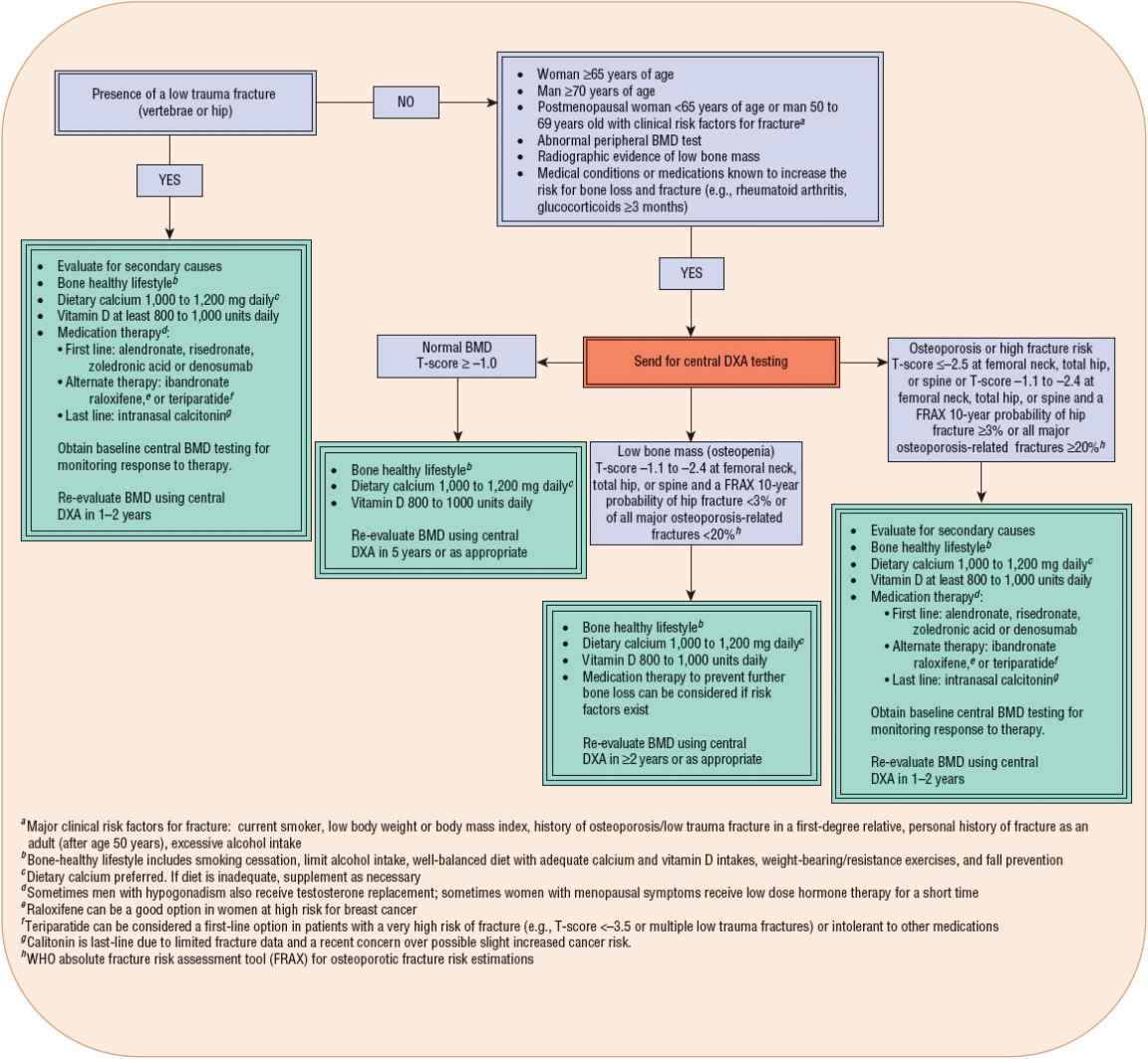
FIGURE 73-3 Algorithm for the management of osteoporosis in postmenopausal women and men aged 50 and older.1,6–8 (BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; FRAX, WHO absolute fracture risk assessment tool; WHO, World Health Organization.)
Nonpharmacologic Therapy
![]() Nonpharmacologic therapy, referred to as a bone-healthy lifestyle, includes proper nutrition, moderation of alcohol intake, smoking cessation, exercise, and fall prevention. A bone healthy lifestyle that is employed early in life will help to optimize peak bone mass and if continued throughout life, it will minimize bone loss over time. Not only does a bone healthy lifestyle target BMD, but it also contributes to decreasing the risk of falls and fragility fractures.
Nonpharmacologic therapy, referred to as a bone-healthy lifestyle, includes proper nutrition, moderation of alcohol intake, smoking cessation, exercise, and fall prevention. A bone healthy lifestyle that is employed early in life will help to optimize peak bone mass and if continued throughout life, it will minimize bone loss over time. Not only does a bone healthy lifestyle target BMD, but it also contributes to decreasing the risk of falls and fragility fractures.
Diet
Overall, a diet well balanced in nutrients and minerals and limited in salt, alcohol, caffeine, and excessive protein is important for bone health.1,6,46 Certain nutrients are emerging to have direct and indirect effects on bone.26,46,47 Being thin or having anorexia nervosa are well known to decrease bone mass. In the past, obesity was thought protective because of increased estrogen production and stimulating bone remodeling due to weight bearing; however, emerging literature suggests leptin and adipose have negative impacts on bone health.26,48
Calcium ![]() Data clearly indicate that adequate calcium intake is necessary for the development of bone mass during growth and for its maintenance throughout life.2,55 Adequate calcium intake is an essential component of all osteoporosis prevention and treatment strategies. Table 73–46–8,45 summarizes the recommended intakes for calcium based on age.27 This value represents the amount needed for 97.5% of the population; higher amounts might be needed when concomitant diseases and medications negatively affect calcium homeostasis. Using calcium-containing foods, which also contain other essential nutrients, is the preferred method to achieve daily calcium requirements. Milk and other dairy products have the highest amount of calcium per serving and are available in low-fat options.27 Some food sources are absorbed well but have low elemental calcium content (e.g., broccoli). Carbohydrates, fat, and lactose increase calcium absorption whereas fiber, wheat bran, phytates (e.g., beans), oxylates (e.g., spinach, rhubarb), high-protein diets, caffeine, and smoking decrease absorption. To get the same amount of calcium in one 8-ounce (~240 mL) glass of milk, one would need to eat 2.25 cups (~530 mL) of cooked broccoli or 8 cups (~1900 mL) of cooked spinach.
Data clearly indicate that adequate calcium intake is necessary for the development of bone mass during growth and for its maintenance throughout life.2,55 Adequate calcium intake is an essential component of all osteoporosis prevention and treatment strategies. Table 73–46–8,45 summarizes the recommended intakes for calcium based on age.27 This value represents the amount needed for 97.5% of the population; higher amounts might be needed when concomitant diseases and medications negatively affect calcium homeostasis. Using calcium-containing foods, which also contain other essential nutrients, is the preferred method to achieve daily calcium requirements. Milk and other dairy products have the highest amount of calcium per serving and are available in low-fat options.27 Some food sources are absorbed well but have low elemental calcium content (e.g., broccoli). Carbohydrates, fat, and lactose increase calcium absorption whereas fiber, wheat bran, phytates (e.g., beans), oxylates (e.g., spinach, rhubarb), high-protein diets, caffeine, and smoking decrease absorption. To get the same amount of calcium in one 8-ounce (~240 mL) glass of milk, one would need to eat 2.25 cups (~530 mL) of cooked broccoli or 8 cups (~1900 mL) of cooked spinach.
TABLE 73-4 Recommended Dietary Allowances and Upper Limits of Calcium and Vitamin D
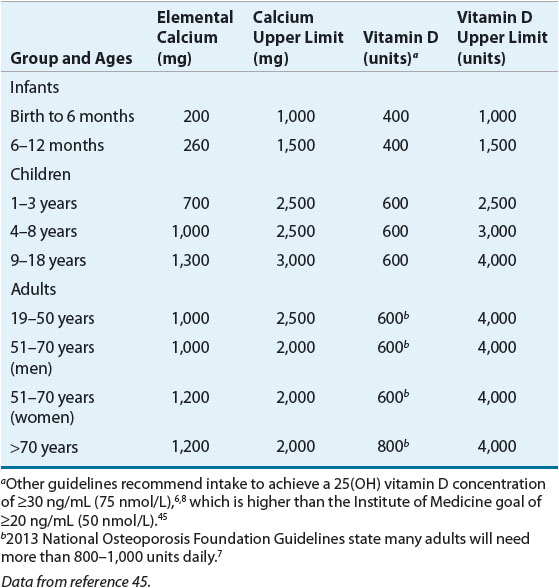
People should be encouraged to evaluate their food and beverage intake to determine if they are receiving adequate intakes. To calculate the amount of calcium in a serving of food, consumers can add a zero to the percentage of the daily value listed on food labels. For example, a serving of milk (8 oz. [~240 mL]) has 30% of the daily value of calcium. This translates to 300 mg calcium per serving.
Approximately 25% of the U.S. population has some level of lactose intolerance, with the incidence in Asian (80%) and African American (50%) populations being much higher than in whites (10%).57 Lactose-intolerant patients have several options, including products containing lactase (Lactaid), lactose-reduced milk, lactose-free milk, calcium-fortified soy milk, certain aged cheeses, or yogurt with active cultures along with other nondairy calcium-fortified products (e.g., orange juice, breakfast cereals, and energy bars).
Vitamin D ![]() Table 73–4 lists the recommended adequate intakes for Vitamin D.45 The three main sources of vitamin D are sunlight (cholecalciferol, vitamin D3), diet, and supplements.42,43 Vitamin D3 comes from oily fish, eggs, and fortified dairy products. Vitamin D2 comes from fungi and eggs. Websites can be used to identify the few foods high in vitamin D.61 To calculate the amount of vitamin D in a serving of food, multiply the percent daily value of vitamin D listed on the food label by 4 (e.g., 20% vitamin D = 80 units).
Table 73–4 lists the recommended adequate intakes for Vitamin D.45 The three main sources of vitamin D are sunlight (cholecalciferol, vitamin D3), diet, and supplements.42,43 Vitamin D3 comes from oily fish, eggs, and fortified dairy products. Vitamin D2 comes from fungi and eggs. Websites can be used to identify the few foods high in vitamin D.61 To calculate the amount of vitamin D in a serving of food, multiply the percent daily value of vitamin D listed on the food label by 4 (e.g., 20% vitamin D = 80 units).
Inadequate concentrations of vitamin D are common in all age groups, especially in older adults and individuals who are malnourished or obese, live in an institution (e.g., nursing home), or live in more northern latitudes. Low vitamin D concentrations result from insufficient intake, dietary fat malabsorption, decreased sun exposure, decreased skin production, or decreased liver and renal metabolism. Endogenous synthesis of vitamin D can be decreased by factors that affect exposure to or decrease skin penetration of ultraviolet B light. Sunscreen use, full body coverage with clothing (e.g., women wearing veiled, full-length dresses), and darkly pigmented skin can all cause a decrease in vitamin D production. Seasonal variations in vitamin D concentrations are also seen with nadirs in late winter and peaks in late summer. Because few foods are naturally high or fortified with vitamin D, most people, especially older adults, require supplementation.
Other Nutrients and Minerals Vitamin K is a cofactor for carboxylation (activation) of proteins, such as osteocalcin, which are involved in bone formation.1,13 Vitamin K deficiency can contribute to bone loss and increase fracture risk. Although some vitamin K supplement studies in osteoporosis reported reduced bone loss and fracture risk, data are conflicting and insufficient to recommend routine supplementation. Patients on warfarin should either use calcium products without vitamin K or use supplements with vitamin K consistently after discussion with their healthcare provider. Insufficient to no data exist for routinely using or supplementing other nutrients and minerals such as potassium, boron, magnesium, and vitamins B, C, and E.1,13 Nutrition research continues to identify more foods that improve bone health, potentially by inhibiting oxidative stress’s negative impacts on bone remodeling, increasing BMP and Wnt signaling to increase bone formation, and altering the conversion of mesenchymal cells via PPAR-γ from adipocytes to osteoblasts.26
Isoflavones Isoflavone phytoestrogens are plant-derived compounds that possess weak estrogenic agonist and antagonist effects throughout the body.49 The most common source for isoflavones is dietary soy products. Genistein is the most abundant and biologically active isoflavone in soybeans. Isoflavones are also available as a supplement or part of a calcium combination product. The evidence supporting a positive bone benefit from isoflavone (soy protein or supplements) intake is conflicting1,49; however, meta-analyses of randomized placebo-controlled studies have found foods and supplements with at least 75 mg isoflavones increased spine, but not hip, BMD when compared with placebo.50 Isoflavones from soy foods appear safe, but more information is needed, especially in women with breast cancer and regarding use from supplements.
Alcohol ![]() Excessive, but not moderate, alcohol consumption is associated with an increased risk for osteoporosis or fractures.1,26 Alcohol increases bone resorption by increasing RANKL and decreases bone formation by inhibiting Wnt signaling pathway and increasing oxidative stress that results in osteoblast apoptosis. Patients with alcohol problems might also have poor nutrition and have balance impairments resulting in more falls and fractures. Alcohol consumption should not exceed one drink per day for women1 and two drinks per day for men.6
Excessive, but not moderate, alcohol consumption is associated with an increased risk for osteoporosis or fractures.1,26 Alcohol increases bone resorption by increasing RANKL and decreases bone formation by inhibiting Wnt signaling pathway and increasing oxidative stress that results in osteoblast apoptosis. Patients with alcohol problems might also have poor nutrition and have balance impairments resulting in more falls and fractures. Alcohol consumption should not exceed one drink per day for women1 and two drinks per day for men.6
Caffeine ![]() Although results are conflicting, excessive caffeine consumption is associated with increased calcium excretion, increased rates of bone loss, and a modestly increased risk for fracture.46 Ideally, caffeine consumption should be limited to two servings or less per day. For those with greater intakes, the increased calcium excretion might be compensated by an additional 40 mg calcium intake for each cup of caffeine-containing beverage.
Although results are conflicting, excessive caffeine consumption is associated with increased calcium excretion, increased rates of bone loss, and a modestly increased risk for fracture.46 Ideally, caffeine consumption should be limited to two servings or less per day. For those with greater intakes, the increased calcium excretion might be compensated by an additional 40 mg calcium intake for each cup of caffeine-containing beverage.
Smoking
![]() Counseling patients of all ages on smoking cessation can help to optimize peak bone mass, minimize bone loss, and ultimately reduce fracture risk.51 Cigarette smoking is an independent risk factor for osteoporosis and is associated with up to an 80% increased relative risk for hip fracture.2 The effect is dose and duration dependent. The negative bone effects resulting from poor nutrition and/or lower 25(OH) vitamin D concentrations are associated with reduced intestinal calcium absorption, an increase in bone resorption from a decrease in production and increase in metabolism of estradiol, increase in RANKL and decrease in OPG, decrease in osteoblasts and bone formation secondary to increase in cortisol and dehydroepiandrosterone sulfate (DHEA-S), and impairment of osteoid production and mineralization. The detrimental effects of smoking on neuromuscular function and balance may contribute to an increased risk of falls.
Counseling patients of all ages on smoking cessation can help to optimize peak bone mass, minimize bone loss, and ultimately reduce fracture risk.51 Cigarette smoking is an independent risk factor for osteoporosis and is associated with up to an 80% increased relative risk for hip fracture.2 The effect is dose and duration dependent. The negative bone effects resulting from poor nutrition and/or lower 25(OH) vitamin D concentrations are associated with reduced intestinal calcium absorption, an increase in bone resorption from a decrease in production and increase in metabolism of estradiol, increase in RANKL and decrease in OPG, decrease in osteoblasts and bone formation secondary to increase in cortisol and dehydroepiandrosterone sulfate (DHEA-S), and impairment of osteoid production and mineralization. The detrimental effects of smoking on neuromuscular function and balance may contribute to an increased risk of falls.
Exercise
![]() Physical activity or exercise is an important nonpharmacologic approach to preventing osteoporotic fractures. Exercise can decrease the risk of falls and fractures by stabilizing bone density and improving muscle strength, coordination, balance, and mobility.2 Physical activity is especially important early in life as lack of exercise during growth can lead to suboptimal loading/straining, decreased stimulation of bone deposition, and a subsequently reduced peak bone mass. All patients who are medically fit should be encouraged to perform a moderate-intensity weight-bearing activity (e.g., walking, jogging, golf, stair climbing) daily and a resistance activity (e.g., weight machines, free weights, or elastic bands) at all ages.7 For men, guidelines specifically suggest those at risk of osteoporosis participate in weight-bearing activities three to four times weekly for 30 to 40 minutes per session.6
Physical activity or exercise is an important nonpharmacologic approach to preventing osteoporotic fractures. Exercise can decrease the risk of falls and fractures by stabilizing bone density and improving muscle strength, coordination, balance, and mobility.2 Physical activity is especially important early in life as lack of exercise during growth can lead to suboptimal loading/straining, decreased stimulation of bone deposition, and a subsequently reduced peak bone mass. All patients who are medically fit should be encouraged to perform a moderate-intensity weight-bearing activity (e.g., walking, jogging, golf, stair climbing) daily and a resistance activity (e.g., weight machines, free weights, or elastic bands) at all ages.7 For men, guidelines specifically suggest those at risk of osteoporosis participate in weight-bearing activities three to four times weekly for 30 to 40 minutes per session.6
Fall Prevention
![]() Risk of falling increases with advanced age predominantly as a result of balance, gait, and mobility problems, poor vision, reduced muscle strength, impaired cognition, multiple medical conditions (e.g., arrhythmias, postural hypotension, Alzheimer’s dementia, Parkinson disease), and polypharmacy.52 Psychoactive medications such as benzodiazepines, antidepressants, antipsychotics, sedative–hypnotics, and opioids have been strongly associated with falls.
Risk of falling increases with advanced age predominantly as a result of balance, gait, and mobility problems, poor vision, reduced muscle strength, impaired cognition, multiple medical conditions (e.g., arrhythmias, postural hypotension, Alzheimer’s dementia, Parkinson disease), and polypharmacy.52 Psychoactive medications such as benzodiazepines, antidepressants, antipsychotics, sedative–hypnotics, and opioids have been strongly associated with falls.
The ability to adapt to falls also decreases with aging. Older adults are more likely to sustain a hip or pelvic fracture because they tend to fall backward or sideways instead of forward.33
Because of the link between falls and fractures, all older adults should be asked whether they have experienced a fall within the last year and about difficulties with walking or balance. Older adults who experience an acute fall requiring medical treatment or those who report or are observed with difficulty with balance or walking should have a multifactorial fall risks assessment and potentially an intervention program.46,52 Multi- versus single-intervention programs have greater effects on decreasing falls, fractures, other injuries, and nursing home and hospital admissions.
Patients should be educated on personal and home safety options to decrease falls.7,52 Websites provide great patient education materials with solutions for commonly observed safety problems.32,53,54 Medication profiles should also be reviewed for any unnecessary medications that can affect cognition and balance and potentially increase fall risk. Consideration should be given to replacing high-risk medications with safer alternatives. Vitamin D supplementation has been associated with reduced falls.42,43,52 Maintenance of a regular individualized exercise program, such as tai chi, should be recommended to improve body strength, balance, and agility.
Other recommendations include resolving vision, low blood pressure, heart rate and rhythm, and foot problems and using proper footwear.
External hip protectors are specialized undergarments designed to pad the area surrounding the hip, decreasing the force of impact from a sideways fall. Conflicting results and poor adherence limit their use.1,46
Pharmacologic Therapy
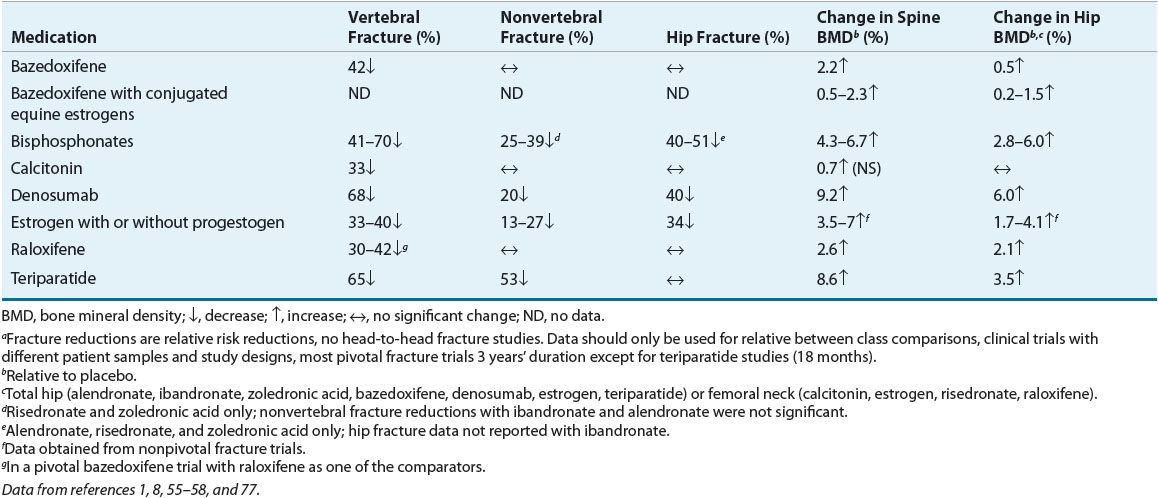
Because nonpharmacologic interventions alone are frequently insufficient to prevent or treat osteoporosis, medication therapy is often necessary. Table 73–51,8,55–58 describes fracture and BMD effects, Table 73–6 describes dosing of osteoporosis medications and Table 73–7 outlines adverse effects and monitoring. These medications should always be combined with a bone-healthy lifestyle.
TABLE 73-5 Fracture and Bone Mineral Density Effects of Osteoporosis Medications from Pivotal Fracture Trialsa in Postmenopausal Women