97
CHAPTER OUTLINE
■ HISTORICAL PERSPECTIVE ON OPIOIDS
■ PREVALENCE OF USE AND MISUSE OF PRESCRIPTION OPIOIDS
■ POSITION OF OPIOIDS IN THE TREATMENT OF PAIN
■ SPECIAL ISSUES IN THE USE OF OPIOIDS
■ CLINICAL VARIABLES IN THE USE OF OPIOIDS
■ PAIN MANAGEMENT IN PERSONS WITH ADDICTION
Opioids are the most potent analgesic agents clinically available at this time. They have wide efficacy and utility in the treatment of acute and cancer-related pain and may be helpful as a component of the management of chronic non–cancer-related pain in some patients. With appropriate care, they may be used effectively and safely in persons with addiction disorders. However, opioids may cause euphoria or “reward” in some individuals, which may lead to misuse and, in susceptible individuals, to addiction. This chapter addresses key conceptual, pharmacologic, and clinical issues related to opioids as a basis for weighing the potential benefits and risks of their use in the treatment of pain, and the chapter presents clinical strategies for safe and effective use in persons with, or at risk for, substance use disorders (SUDs). To provide context, we begin with brief discussion of the historical use of opioids, the prevalence of pain and opioid use and misuse in our society, and the contemporary views on the use of opioids in the treatment of pain.
HISTORICAL PERSPECTIVE ON OPIOIDS
Opioids have been used for medicinal purposes for millennia. One of the first documentations of the use of opioids comes in the Sumerian ideogram of hul gil, the “plant of joy,” inscribed more than 5,000 years ago. Theophrastus, the Greek philosopher and popularizer of science, provides the first written account of the use of opium to relieve pain in 300. During this time, the Greek physicians Hippocrates and Galen used opium to treat headaches, coughing, asthma, and melancholy. In 1805, morphine was purified from opium, but use did not become widespread until the development of the hypodermic syringe in 1853, which allowed morphine to be introduced directly into the circulatory system. Heroin was introduced as an over-the-counter drug by the Bayer Company in 1895 and widely marketed as a panacea for numerous medical conditions (1).
By the early 1900s, both appropriate therapeutic use and misuse of opioids were widespread in the United States. The Institute of Medicine estimates that about 300,000 Americans were opioid addicted at that time: With a total population of a little over 75,000,000 in 1900, this would be about 1 in every 250 people (2). The medical community came to recognize the problem of opium addiction, and in a 1900 article in the Journal of the American Medical Association, Dr. John Witherspoon, referring to opioids, exhorted physicians to “save our people from the clutches of this hydra-headed monster which stalks the civilized world, wrecking lives and happy homes, filling our jails and lunatic asylums…” (3).
Opioids were subsequently subjected to regulation and taxation through the Harrison Narcotics Tax Act in 1914, with legal use restricted to “legitimate medical purposes.” In 1919, a federal ruling held that treatment of addiction was “outside the realm of legitimate medical interest” (4), creating a conundrum that allowed physicians to treat pain but not addiction that sometimes occurred in the context of medical use (5). Prescribing of opioids decreased and remained relatively low into the 1960s. An article on managing cancer pain in the JAMA in 1941 reflects the low esteem in which opioids were held, “The use of narcotics in the terminal cancer patient is to be condemned … due to undesirable side effects … dominant in the list of these … is addiction” (6). Through this dark period, a diagnosis of cancer struck terror in peoples’ hearts, and elective surgeries were deferred until the problem was more unbearable than anticipated postoperative pain.
The hospice and palliative care movement blossomed in England in the mid-1960s under the leadership of Dame Cicely Saunders at St. Christopher’s hospice. With demonstration that opioid use could be safe, effective, and provide comfort at the end of life, the movement spread rapidly to the United States (7). With observation of favorable outcomes in hospice patients, aggressive management of acute pain emerged. The 1970 Federal Controlled Substances Act classified controlled substances into risk categories and required registration of providers; this provided a structure that both supported and allowed control of use of opioids and other controlled substances. When cancer pain specialists noted favorable long-term results from opioid therapy of pain in cancer survivors without the inevitable evolution of tolerance or addiction, they began to use opioids for serious non–cancer-related chronic pain and published a case series that spread interest in the use of opioids for all types of pain (8).
In the early 1990s, controlled-release opioids were introduced and provided a new tool that many believed would improve pain management by providing more stable blood levels and thus more consistent relief. Several national initiatives also promoted more aggressive pain management: The U.S. Department of Health and Human Services published Guidelines to Treatment of Cancer Pain and Treatment of Acute Pain (9,10); in 2001, the Joint Commission on Accreditation of Hospital Organizations implemented evaluation and management of pain as a quality measure (11); and in 2000, the U.S. Veterans Administration declared pain the fifth vital sign (12). With the convergence of all these factors, an era of aggressive pain management was born, and opioids became increasingly central to pain control.
PREVALENCE OF PAIN
Pain is one of the most common ailments for which patients seek medical care. According to a 2011 report of the Institute of Medicine, persistent pain affects 100 million Americans at a cost of about 600 billion dollars a year when lost productivity and medical costs are combined; these numbers are expected to grow as baby boomers age (13). The most commonly reported pain disorders are headache, lower back pain, arthritis, and other joint pain (14). Pain is the second leading cause of workplace absenteeism (15). A large systematic review of pain following traumatic musculoskeletal injury found that a large proportion (28% to 93%) of patients experiencing traumatic musculoskeletal injury will develop persistent pain for a period of up to 84 months, although the severity of pain generally decreases over time (16). The prevalence of pain among persons with SUDs may be significantly higher than that of the general population with studies suggesting pain occurring in 62% to 80% of patients in methadone maintenance (MMT) treatment programs and 78% of patients presenting for inpatient substance abuse treatment programs reporting significant chronic pain (17,18). Persons with alcohol and other SUDs are vulnerable to traumatic injury (19,20), and this may contribute to a relatively higher prevalence of chronic pain in persons with SUDs.
PREVALENCE OF USE AND MISUSE OF PRESCRIPTION OPIOIDS
Opioid use and misuse and opioid-associated harm have increased in parallel over the past decade and a half. As opioids have been increasingly prescribed for pain, opioid medications sold through prescription channels intended for therapeutic use increased fourfold between 1999 and 2010 (21). In a similar time frame, from 2000 to 2010, the treatment admission rate for dependence on opiates other than heroin as a primary diagnosis increased over 400% for persons aged 12 and older (22). Between 2004 and 2010, emergency room (ER) visits related to prescription opioids increased almost 250% (23). Opioid overdose deaths nationally also quadrupled from 1999 to 2010 (21). In the aggregate, these data suggest that the more opioids that are available for pain, the more are available for misuse and associated harm.
While preclinical thefts of opioids (e.g., from pharmacies, manufacturers, and trucks) contribute to diversion of opioids (24), evidence suggests 70% of persons who report misuse of opioids divert them from friends or relatives, while about 18% get them directly from doctors. It is important to note that those diverted from friends or relatives are thought to come from a single physician over 80% of the time, so clinical sources are clearly important sources of misused opioids (25). In order to effectively and safely treat pain, while reducing the risk of opioid misuse and diversion, it is critical that prescribing clinicians understand and employ clinical strategies to reduce the risk of diversion of these medications and that patients secure all supplies of opioids.
POSITION OF OPIOIDS IN THE TREATMENT OF PAIN
Opioids are the most potent analgesic agents available. For severe acute postsurgical or trauma-induced pain, opioid therapy is the standard of practice, while nonopioid analgesics and interventions are often used when available and effective. Opioids are also standard in the treatment of advanced or progressive cancer-related pain, occupying steps two and three of the widely accepted “therapeutic ladder” developed by the World Health Organization (WHO) (26) though their availability is limited in many parts of the world (27). There is less consensus, however, regarding the role of opioids in the treatment of chronic noncancer pain or pain not associated with a terminal illness.
Chronic pain often has multiple etiologic components that include physiologic, psychosocial, and behavioral factors, and it may similarly impact diverse biopsychosocial domains of experience. Because of this, effective treatment of chronic pain often demands an interdisciplinary treatment approach that includes empowerment of the individual in management of pain and related symptoms. Such approaches are explored in the accompanying chapters in this section of the textbook. Opioids may be helpful, however, in the management of persistent pain in some individuals for whom nonopioid treatments are not effective or for whom other interventions present greater risk (28). While research is ongoing to develop an evidence base to determine what patient characteristics, medications, dosing regimens, and other variables favor safe and effective opioid use, numerous organizations and state and national agencies have developed guidelines to shape opioid use in patients with chronic non–cancer-related pain (29–34). As cancer treatment has improved and cancer for many people has become a chronic illness with long-term survival, there is ongoing reevaluation of the role of opioids in treatment of long-term nonprogressive cancer-related pain (35). Clinical management of opioids in the treatment of acute pain, pain associated with cancer, and other potentially terminal illness and chronic non–cancer-related pain will be addressed later in this chapter.
SPECIAL ISSUES IN THE USE OF OPIOIDS
Like most medications, opioids have the potential for both benefit and harm. In addition to a range of physical side effects, however, opioids, like other controlled substances, deserve special consideration for their capacity to provide reward (euphoria) and to produce addiction in some persons. These characteristics can lead to their misuse, diversion, and associated harm. In addition, opioids may be associated with pharmacologic effects, including physical dependence, tolerance, and hyperalgesia, which can complicate their use and require special care in management.
These issues may be of less significance in the treatment of acute and cancer pain, given the often time-limited duration of use in these contexts, than in the management of chronic non–cancer-related pain, but a thorough understanding of these issues may enhance decision making in all three contexts. Each issue is considered separately here. Other side effects of opioids, including the critical issues of respiratory depression, are discussed below under side effects.
Physical Dependence
Physical dependence has been defined as a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist (35a). Such dependence is an expected occurrence in all patients (with and without co-occurring addiction) after 2 to 10 days of continuous administration of an opioid (36). In an acute pain setting, such dependence is not usually clinically significant, because individuals tend to taper opioids naturally owing to gradual reduction in pain as the acute problem (such as postoperative pain, posttraumatic pain, or medical illness) resolves. However, if pain medications are abruptly stopped or precipitously reduced, a withdrawal syndrome may ensue. The character and intensity of the withdrawal vary, depending on the dose and duration of opioid administration and a variety of host factors, including previous experience with withdrawal, prior long-term administration of opioids, and the patient’s expectations regarding withdrawal.
Common symptoms of opioid withdrawal include autonomic signs and symptoms, such as diarrhea, piloerection, sweating, mydriasis, and mild increases in blood pressure and pulse, as well as signs of central nervous system (CNS) arousal such as irritability, anxiety, and sleeplessness. Craving for the medication is expected in the course of withdrawal, and pain—most often experienced as abdominal cramping, deep bone pain, or diffuse muscle aching—is common (37). Patients with chronic pain may experience an intensified level of their usual pain syndrome during withdrawal. In patients who are physically dependent on opioids, the use of short-acting opioids may result in intermittent withdrawal between doses, which may cause an increase in perceived pain (38). This may be avoided by using long-acting or continuous medications (39). Simple physical dependence occurs in most patients when opioids are administered for an extended period of time.
Using the term addiction to describe physical dependence is inaccurate and raises misperceptions about both persons with addiction disorders who become physically dependent on medications despite continuing in a state of recovery and individuals without addiction disorders who become physically dependent on medications without developing the true characteristics of addiction.
Tolerance
Tolerance is indicated by the need for increasing doses of a medication to achieve the initial effects of the drug (40,41). Tolerance may occur both to a drug’s analgesic effects and to such side effects as respiratory depression, sedation, or nausea. Tolerance can be innate due to inherent biogenetic characteristics of the individual (in response the particular opioid), or it can be acquired in response to ongoing exposure to the opioid. Acquired tolerance may be due to both pharmacokinetic factors, such as changes in drug absorption and metabolism that reduce blood concentration, and to pharmacodynamic factors such as receptor desensitization or other density changes at the level of opioid receptors (42). Changes in central immune signaling and in N-methyl-D-aspartate (NMDA) receptor activity are involved in the evolution of tolerance (43). There is currently burgeoning research into the molecular mechanisms of tolerance.
The patterns of occurrence of tolerance in clinical settings have not been well described. Animal studies suggest that tolerance to the analgesic effects of medications occurs in some contexts but not in others (42). Human studies of the management of acute pain document the development of progressive tolerance to the analgesic effects of opioids when they are administered on a continuous basis over a period of several days (44,45). Over a period of weeks to months, however, some studies suggest that the continuing development of progressive tolerance to the analgesic effects of opioids may not routinely occur; older studies that examined the opioid management of cancer pain suggest that opioid dose requirements increase only during progression of the underlying disease process and that, with stable disease or treatment of painful tissue pathology, the need for medication remains the same or actually decreases (41,46). An 18-month randomized controlled clinical trial supports the clinical observation that stable opioid doses may provide persistent, if modest, improvement in analgesia over the longer term in some patients with chronic noncancer pain as well (47), though other studies suggest progressive gradual tolerance does occur in chronic pain in many patients (48).
A number of studies investigated the development of tolerance to specific opioids, with the goal of determining whether opioids of varying efficacy have different profiles with regard to the development of tolerance. Several animal studies have suggested an inverse relationship between analgesic efficacy and tolerance formation; however, no consistent relationship between intrinsic efficacy and tolerance has been demonstrated in human populations (45,49,50).
While absolute tolerance to the analgesic effects of opioids does not appear to be a critical limiting factor to the treatment of acute pain or cancer-related pain in most patients, in the context of chronic pain not associated with life-threatening illness, there is increasing evidence of diminishing returns and potential harm associated with high-dose opioid use, and thus progressive tolerance may be a practical factor in long-term use of opioids in this context (see discussion of opioid dosing and management of tolerance in Chronic Nonterminal Pain). Research to better understand and prevent tolerance will be important to improving opioid outcomes.
Hyperalgesia
Opioid use may increase pain in some contexts. Observational studies have long indicated that some individuals with pain who use opioids on a long-term basis experience improvement in pain after tapering or simple withdrawal of opioids, without the institution of other major pain interventions (51–55). These studies include observations of patients with pain and opioid dependence in both pain treatment and addiction treatment settings. Though higher levels of pain on opioids in some of these studies may have been mediated by intermittent withdrawal-induced hyper-algesia associated with short-acting opioids, it is increasingly appreciated that opioids may result in hyperalgesia as well.
Hyperalgesia is a physiologic state in which there is sensitization to nociceptive stimuli resulting in lowered pain threshold and/or decreased tolerance to pain. Extensive experimental evidence demonstrates that at the same time opioids provide potent analgesia, they paradoxically set in motion molecular processes that eventually result in opioid-induced hyperalgesia (OIH) (56). OIH has been well demonstrated in both animals and humans (57–59).
The mechanisms of hyperalgesia are not fully defined but appear to involve both glutamate-mediated activation of NMDA receptors and induction of inflammatory cytokines in the CNS (60,61). It is clear that similar systems are involved in the evolution of OIH and of opioid tolerance; however, the clinical and neurophysiologic relationships between opioid tolerance and OIH are not clear.
The extent to which hyperalgesia occurs in clinical settings and its relevance for patients who use opioids on a long-term basis is not known. While hyperalgesia is not clinically apparent in many patient maintained on opioids for months to years, one study demonstrated hyperalgesia after 1 month of controlled-release oral morphine for low back pain (62). A structured evidence-based review concluded there was insufficient evidence to determine whether clinically significant OIH occurs in the context of long-term opioid use in clinical settings (63). More study is needed to better understand the relevance of experimental findings to clinical practice.
Reward
Commonly used analgesic opioids act primarily on mu opioid receptors. Mu opioids produce reward (euphoria) in many, though not all, individuals (64). Some persons, in fact, experience dysphoria or no mood changes in association with opioid use. Kappa opioids (such as butorphanol, pentazocine, or nalbuphine) can produce euphoria, though do so less commonly than mu opioid agonists, and are more often associated with dysphoria (65). The potential for opioids to produce reward when used for pain is a critical factor to consider, however, especially when treating persons with co-occurring pain and SUDs or risks for substance misuse. A clear understanding of the potential issues that may modulate reward experiences when opioids are used for analgesia may be helpful to optimize clinical management strategies, particularly in persons at risk for substance misuse.
Mechanisms of Reward
Human brain reward systems are complex and discussed in more detail in Section 1 of this textbook. One critical step through which opioids produce reward is by binding to GABAergic interneurons that inhibit dopamine production in the limbic reward system, preventing them from doing so (66). The resulting increase in dopamine and cascade of secondary effects produces feelings of reward or euphoria. Reward may or may not occur when opioids are used for pain and is not in itself harmful. In some contexts, particularly terminal illness, euphoria and reward may in fact be valued side effects. However, when euphoria becomes the focus of the use of opioids, particularly in a person with addictive disease, it may undermine pain treatment and become a problem in its own right (67). Understanding how to limit an opioid’s rewarding effects therefore may sometimes be helpful.
Most of what is known about the mechanisms that determine reward intensity derives from addiction literature. This knowledge may, however, also apply and provide some guidance in clinical pain treatment settings.
Factors Affecting Reward
Rate of Increase (Tmax)
Both animal drug self-administration studies and human drug-liking studies have demonstrated that reward increases when the rate of rise in drug blood levels increases: The faster the onset, the better the rush or high (68). One study that examined the properties of prescription opioids that make them more attractive for getting high supported speed of onset as a key value (69). The intravenous (IV) route of administration causes a more rapid rise in blood levels and therefore provides more euphoria than the oral route for a given drug at a given dose, with intramuscular and subcutaneous administration providing intermediate effects (Fig. 97-1). Among opioids given orally, those with an inherently slower time to peak effect (such as methadone or levorphanol) are expected to produce weaker reward effects than opioids with relatively rapid onset, such as immediate-release oxycodone (in Percocet or Tylox), hydrocodone (in Vicodin or Lortab), or hydromorphone (Dilaudid). Rapid-onset transmucosal formulations of fentanyl would be expected to have higher reward than fentanyl used transdermally.
Peak Blood Levels Attained (Cmax)
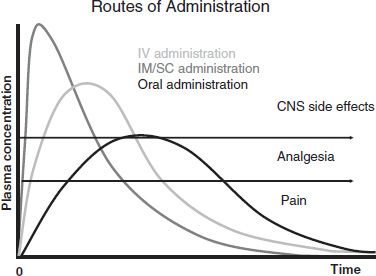
FIGURE 97-1 Routes of administration. IV, intravenous; IM/SC, intramuscular/subcutaneous; CNS, central nervous system.
The higher the opioid blood level relative to the individual’s tolerance for the drug, the greater the reward. An opioid-naïve individual with little or no tolerance may experience euphoria with a drug blood level that would cause no significant reward effect in a more tolerant individual. A dose of an opioid given intravenously achieves a higher peak blood level than the same dose administered by the oral route, with subcutaneous and intramuscular again intermediate (Fig. 97-1).
Changes in Blood Levels
As the rise in blood levels of a drug is associated with onset of euphoria, stable levels generally produce less euphoria than intermittently rising and falling levels. This key principle underlies the effectiveness of MMT therapy for opioid addiction. Based on this principle, in pain treatment, continuous IV infusion of an opioid is expected to trigger less reward than intermittent boluses, and controlled-release opioids (used by the intended route at the intended time interval) or intrinsically long-acting opioids (such as methadone or levorphanol) should be less rewarding than frequently dosed short-acting medications (Fig. 97-2). An exception to this rule is patient-controlled analgesia (PCA), which is not expected to provide significant reward because the incremental doses are very small and spaced at intervals that do not permit a rapid, high rise in blood levels. However, when persons with an active addiction to opioids receive PCA or any IV infusion, close supervision is critical to ensure that no tampering with the system occurs. Based on the increased reward associated with rapid onset of opioids, it is not surprising that many persons who use opioids to get high or due to addiction alter the delivery system by crushing tablets and snorting or injecting the contents to increase reward (70).
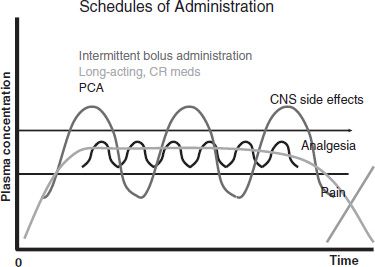
FIGURE 97-2 Schedules of administration. CR, controlled release; PCA, patient-controlled analgesia; CNS, central nervous system.
Mu Receptor Polymorphism
As previously noted, mu opioid agonists are more likely to cause reward than kappa opioid agonists. Emerging understanding of mu opioid receptor polymorphism with variable expression of receptors in different individuals and of the differential effects of different mu agonists on different subreceptor types suggests that contrasting reward and analgesic effects likely occur when different drugs are used by different individuals (71). This may explain why patients treated for opioid addiction may have different opioids of choice and why patients with pain report variable analgesia and side effects with different medications.
Interference of Pain with Reward
Some research in both human and animal models suggests that opioids are less rewarding in the presence of pain (72,73). This hypothesis is supported by reports from patients who say that opioids relieve their pain without psychic effects. As well, many patients experience dysphoria or other aversive feelings, rather than euphoria, when given opioids for their pain.
Though concerns regarding drug reward may be relevant over time in treating persons at risk for opioid misuse or addiction, they should not deter effective and immediate treatment when opioids are required for acute pain. The long-term outcome of addiction is not likely to be strongly affected by use of indicated medications in the short term (74).
Addiction
In the context of pain treatment with opioids, addiction must be defined through the observation of a constellation of maladaptive behaviors rather than by observation of pharmacologic phenomena such as dependence, tolerance, and dose escalation. Addiction in the context of opioid therapy for pain has been characterized by the presence of a combination of observations suggesting adverse consequences due to use of the drugs, loss of control over drug use, and preoccupation with obtaining opioids despite the presence of adequate analgesia (75). Physical dependence on opioids and the development of tolerance to their effects do not, of themselves, constitute addiction (76,77).
Adverse consequences that are suggestive of addiction include persistent sedation or euphoria, deteriorating function despite relief of pain, or increase in distresses such as anxiety, sleep disturbance, or depressive symptoms. Loss of control over use might be reflected in exhausting prescriptions before the expected renewal date, obtaining prescriptions from multiple sources, or obtaining opioids from illicit sources. Preoccupation with opioid use may be reflected in noncompliance with nonopioid components of pain treatment, inability to recognize nonnociceptive components of pain, and the perception that no interventions other than opioids have any effect on pain (75,76). It is important to recognize that such behaviors may occur on an occasional basis for a variety of reasons in the context of successful opioid therapy for pain. By contrast, a pattern of persistent occurrences should prompt concern and further assessment (Table 97-1).
TABLE 97-1 DEFINITION AND INDICATORS OF ADDICTION IN PAIN PATIENTS
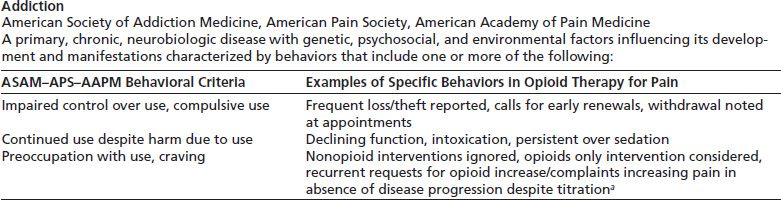
a May reflect tolerance or hyperalgesia.
ASAM, American Society of Addiction Medicine; APS, American Pain Society; AAPM, American Academy of Pain Medicine.
Savage SR, Joranson DE, Covington EC, et al. Definitions related to the medical use of opioids: evolution towards universal agreement. J Pain Symptom Manage 2003;26(1):655–667.
The risk of developing addiction to opioids in the course of opioid therapy for pain is unknown. Early twentieth century studies led to a perception that the iatrogenic creation of addiction through medical use of opioids was a very frequent occurrence (78,79). In contrast, a number of retrospective surveys in the 1980s of never-addicted medical patients suggested that the development of addiction in the course of long-term opioid therapy for pain was almost negligible (80,81), leading to a likely underappreciation of the risks. The reality is almost certainly somewhere in between, but the actual rate of occurrence is difficult to assess.
Studies of the incidence of addiction in the context of pain treatment have generally examined a wide variety of “aberrant behaviors” rather than specifically assessing for addiction, and they have been variable in methodology, operative definitions, and quality. In addition, many studies that assess opioid use and misuse excluded the highest-risk group, those with a prior history of addiction (82).
The prevalence of identified misuse of opioids by patients ranges widely in different studies, between 1% and 38% (83–86). Misuse of opioids, however, can reflect a wide variety of issues as well as addiction. One study of persons presenting for treatment of oxycodone addiction found that 47% (51 of 109) of persons had their first exposure to opioids through a prescription for pain and further found that only 31% of this subgroup had prior histories of alcohol or other drug problems, suggesting that a significant number developed de novo addiction to opioids prescribed for pain in the absence of previous addiction disorder (70). However, the study did not assess for family history of addiction and, therefore, did not shed light on whether such addiction occurred in the context of identifiable biogenetic risk. It also did not provide a denominator that would permit determination of the rate of addiction among those prescribed opioids.
A 2008 structured clinical review sought to estimate the risk of development of a clinically significant SUD, either addiction or abuse, in the course of longer-term opioid therapy of pain and to distinguish these from misuse behaviors per se (87). The review identified 67 scientifically acceptable studies of opioid use and misuse and divided them into those that excluded persons with history of SUD and those that included them and then looked at studies that identified “aberrant behaviors” versus those in which clinicians more formally identified abuse or addiction (SUDs). The review found that across these studies, the risk of de novo opioid SUD in patients with no history of SUD was 0.19% and the risk of aberrant behaviors was 0.59%. In studies that did not exclude persons with SUDs, the risk of opioid SUD was 3.27%, while the risk of aberrant behaviors was 11.9%. The review also examined five studies that included urine drug screening and found that 20.4% and 14.5%, respectively, had unexpected findings suggesting that actual rates of aberrant behaviors and SUD may be higher than suggested based on behavioral observation and clinical assessment and supporting the value of urine drug screening in identifying misuse or addiction in the context of opioid therapy.
The etiology of addiction in the context of pain treatment likely involves an interplay of genetic vulnerability, drug reward effects, and other host and environmental factors including the presence of pain, of stress, and of the psychosocial context of use. According to the National Epidemiologic Survey on Alcohol and Related disorders, the past 12-month prevalence of substance disorders in the United States is estimated to be 9.35% (with confidence intervals of 8.86 and 9.84) (88). It is reasonable to expect that this portion of the population may be at increased risk for the development of addiction when opioids are used for pain, though the presence of pain may reduce this risk somewhat if pain attenuates reward. Nevertheless, it is appropriate to use special care in implementing opioid therapy in patients who have personal or family histories of alcoholism or other addictions.
When addiction is identified in the course of opioid therapy for pain, it is important to address it aggressively, so that the pain is effectively controlled and to prevent the debilitating sequelae of addiction. Institution of appropriate addiction treatment services, tightening the structure of opioid treatment in order to maintain safety, and involving the patient’s social support system in supporting treatment and recovery are important first steps.
Differential Diagnosis of Opioid Misuse
Patients misuse opioids for a variety of reasons with a wide range of implications (Table 97-2). It is important to distinguish clinically between different causes of opioid misuse in order to address each case appropriately.
TABLE 97-2 DIFFERENTIAL DIAGNOSIS OF MISUSE OF ANALGESIC OPIOIDS

A common cause of misuse is simply misunderstanding how opioids are supposed to be used; clear written instructions, with engagement of support systems for those with cognitive challenges, can reduce this type of misuse. Patients may also misuse opioids to obtain relief from depressed feelings, anxiety, insomnia, or discomforting memories; these patients usually will benefit more from more specific treatments of their co-occurring problems. Patients who have become physically dependent on opioids may continue to use the drugs after the pain has resolved in order to avoid withdrawal symptoms; tapering of opioids can usually eliminate dependency without significant distress.
Some patients misuse opioids electively in order to experience reward or euphoria as discussed earlier. A subset of patients who have a biogenetic vulnerability and who use opioids in a manner that induces euphoria will trigger addiction which can lead to continued compulsive use in a manner that poses significant personal risk. Because of their association with reward and addiction, opioids have significant street value and diversion for profit may occur.
Finally, some patients exhibit distress and engage in behaviors aimed at obtaining more medication because they are experiencing unrelieved pain. The term pseudoaddiction has been used to describe that the inaccurate interpretation of these behaviors in patients who have severe pain has not been effectively treated (89). Such patients may be preoccupied with obtaining opioids because of a need for pain control rather than an addictive drive. Pseudoaddictive behavior can be distinguished from addiction by the fact that, when adequate analgesia is achieved, the patient who is seeking pain relief demonstrates improved function, uses the medications as prescribed, and does not use drugs in a manner that persistently causes sedation or euphoria. Use of the medications as prescribed is an important caveat here, since normalization of behaviors may occur transiently in persons with addiction, for whom prescribed opioids equate to opioid agonist therapy of addiction; however, without needed structure and support for recovery, use of opioids in this context may unravel rapidly into misuse.
Risk Factors for Opioid Misuse
A number of risk factors for opioid misuse in the context of pain treatment are emerging consistently across numerous studies and reviews. According to three recent systematic reviews, perhaps not surprisingly, these include history of mental health disorders, history of SUD, and/or a history of substance-related legal problems (90–92). However, these reviews concur that current evidence cannot clearly determine whether SUDs or mental health conditions preceded or were a consequence of chronic pain and opioid treatment; they agree more research is needed to confirm the suggested associations. In addition, it is noted that risk prediction does not necessarily equate with actual misuse, so recognition of variables associated with increased risk must be integrated with other clinical information in decision making related to opioid prescribing.
Mental Health Issues
The 2002 National Epidemiologic Study of Alcohol and Related Condition showed that mood disorders (including a spectrum of depressive and anxiety disorders) were associated with an elevated risk for prescription opioid misuse and supported a self-medication model of misuse (93). It also found that individuals who misused opioids had an increased risk of developing mood disorders in the absence of preexisting comorbidity.
An analysis of the 2003 National Survey on Drug Use and Health (NSDUH) found that individuals with any co-occurring mental health conditions had twice the risk for initiation of nonmedical use of opioids, and those with depressed feelings for 2 or more weeks had 2.5 times the risk of those with no co-occurring condition (94). The NSDUH study also found that any lifetime use of marijuana, cocaine, or heroin was associated with increased likelihood of nonmedical use of prescription opioids and that Whites had twice the likelihood of African Americans.
A study of patients presenting to an emergency department for a renewal of opioids found that trait anxiety and panic disorder, as assessed using five validated self-report instruments and a structured clinical interview, correlated with a positive screen for risk of opioid misuse using a validated screen for risk of opioid misuse (95).
History of Substance Use Disorder
Prior SUD is clearly a major risk factor for misuse of prescription opioids. Longitudinal administrative data on 15,160 veterans showed that a history of SUD was a strong predictor of opioid misuse, and co-occurring mental health disorder was a moderately strong predictor of recognized prescription opioid abuse and dependence. Because mental health disorders were more prevalent than SUDs (45.3% vs. 7.6%), they accounted for greater attributable risk in the population (96). A more controlled study of 127 veterans prescribed opioids in a primary care setting found that patients with a history of an SUD were three to six times more likely to misuse opioid medications than patients without a history of an SUD (97).
Another study found younger age, history of alcohol abuse or current marijuana or cocaine use, and history of legal problems with alcohol or drugs increased the risk of prescribed opioid misuse (84), and another found that individuals with evidence of cocaine use were much less likely to resolve opioid misuse in a structured prescribing program than other patients (98).
Another found that individuals seeking treatment for prescription opioid addiction were more likely to recall reward effects on their first exposure to opioids than a control group of chronic pain patients on long-term opioid treatment who did not develop addiction (67). Another identified medication craving as a predictor of prescribed opioid misuse (99). Recently, current tobacco use has emerged as a marker for risk of prescribed opioid misuse (99a). These suggest that underlying vulnerability in the limbic reward system may drive prescribed opioid misuse.
Finally, a large study using two large payor databases found a robust correlation between younger age, mental health diagnoses, and history of SUD and the presence of a diagnosis of opioid use disorder. They also described the phenomenon of adverse selection, that is, the fact that persons with mental health and SUDs who are at higher risk for opioid misuse are in fact more likely to be prescribed opioids for pain (100).
CLINICAL VARIABLES IN THE USE OF OPIOIDS
Opioids are often the mainstay of treatment of moderate to severe acute pain and pain associated with progressive or terminal illnesses. When used in the long-term treatment of chronic, nonterminal pain, they are most often helpful as one component of multidimensional treatment. Goals of pain treatment generally include reduction in pain, enhanced level of function, and improved quality of life. Use of opioids in different treatment contexts will be considered later in this chapter.
A number of variables must be considered in planning opioid therapy for pain. In addition to consideration of the special issues addressed above, important variables include drug selection, dose titration and scheduling, and potential side effects. It also is clinically important to understand how to appropriately change drugs or withdraw medications when indicated. These issues are considered individually.
Drug Selection
Opioids produce their analgesia and side effects primarily through action on opioid receptors. Stimulation of the mu, kappa, and delta receptors is associated with analgesia and side effects (101). Most of the commonly used opioid analgesics have predominantly mu receptor activity; however, some analgesic opioids have agonist–antagonist or partial mu agonist activity. Each of these opioid classes is considered here.
Mu opioid agonists are the most commonly prescribed class of opioid analgesics and include morphine, oxycodone, hydromorphone, oxymorphone, methadone, hydrocodone, and fentanyl (Table 97-3). Pure mu agonists usually have no ceiling analgesic effect and may be titrated as needed to achieve analgesia, limited by side effects and risks as discussed elsewhere. Tolerance to some side effects generally occurs more rapidly than tolerance to analgesia, though monitoring for respiratory depression is important, especially in opioidnaïve individuals, as doses are increased or specific opioids are changed.
TABLE 97-3 OPIOIDS COMMONLY PRESCRIBED FOR PAIN WITH KEY CHARACTERISTICS
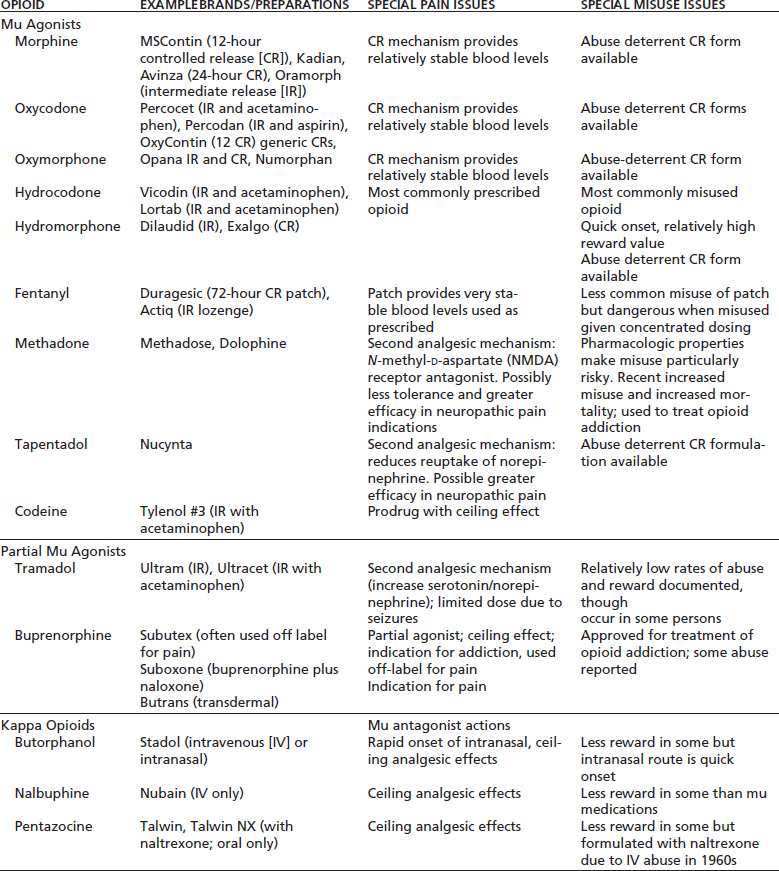
Adapted from Savage SR, Passik S, Kirsch K. Challenges in using opioids to treat pain in persons with substance use disorders. Addict Sci Clin Pract 2008;4(2):4–25.
Though most mu agonists are interchangeable if attention is paid to relative potencies and onset and duration of action, individuals may respond differently to different opioids in terms of both analgesia and side effects. This may be owing in part to variability in mu opioid receptor expression in different individuals as well as to variable opioid receptor specificity of the different drugs (102). Some mu receptor polymorphisms may require higher agonist dosing to achieve maximal analgesic efficacy (103). Additionally, individual differences have been demonstrated in opioid metabolic pathways due to variations in cytochrome 450 enzyme expressions (104). Recognition of variability in both opioid receptor expression and opioid metabolism can explain observed differences in patient responses to different opioids and holds promise for the use of pharmacogenetic testing in matching individuals with opioids that may be most effective for them with least side effects (105).
Some clinically relevant differences between mu agonists long have been apparent. Meperidine’s usefulness in pain treatment is limited by its short half-life and its neurotoxic metabolite, normeperidine, which may accumulate with high-dose therapy, causing irritability, tremors, and, potentially, seizures (106). This is especially relevant in opioid-dependent individuals who may have significant tolerance and correspondingly high-dose requirements as well as in patients with renal or hepatic insufficiency.
Propoxyphene, a weak mu agonist, has low analgesic efficacy and some abuse potential and has been associated with seizures. Some clinicians believe it is a reasonable analgesic substitute for nonsteroidal antiinflammatory drugs (NSAIDs) or acetaminophen in some patients who cannot use those drugs. A subset of patients appear to experience more substantial analgesia with propoxyphene than with NSAIDs. However, most experts agree that propoxyphene has limited utility in pain treatment, and it was withdrawn from FDA approval in the United States in 2010 due to emerging data on cardiac conduction abnormalities (107).
Methadone differs from other mu agonists in several ways, some of which make its lethality if misprescribed or misused greater than that of other opioids. It has a long and unpredictable elimination half-life (5 to 130 hours) that necessitates especially careful titration (108). Its dextro isomer has NMDA receptor antagonist activity, and it has been suggested that this may result in greater efficacy in treating neuropathic pain, though this has not been demonstrated clearly in clinical trials (109).
Methadone is metabolized by the 450 cytochrome system, and its blood levels may be critically affected by other drugs that induce or inhibit this system (110). For example, phenytoin (a cytochrome p450 inducer) may markedly reduce methadone levels causing patients to experience pain or withdrawal, while many antiretroviral agents (cytochrome p450 inhibitors) may cause increased blood levels with risk for sedation or even overdose in some contexts.
Further, incomplete cross-tolerance between methadone and other mu opioid agonists requires the use of a much lower than calculated equianalgesic dose of methadone in the patient who is transitioning from other mu agonists. Guidelines have recommended starting doses as low as 20 to 30 mg/d, despite prior use of other opioids at high doses (111). Conversely, patients who are changed from methadone to other opioids may experience poor analgesia for reasons that are not fully understood (112).
Methadone is known to cause significant QTc prolongation in some patients and has been associated with torsades de pointes syndrome. Some experts recommend EKG prior to starting methadone and in the course of titration, especially for those with special risk factors, and suggest that methadone not be started with QTcs above 450 ms, that discontinuation be considered at QTc between 450 and 500 ms, and that above 500 ms methadone be tapered (113). EKG changes may be of particular significance when methadone is combined with other drugs that prolong QT intervals. Methadone is more difficult to use safely than other opioids owing to its variable half-life and multiple drug interactions.
Another mu opioid agonist, levorphanol, is less well studied than methadone but shares a number of its pharmacologic features, including its relatively long half-life and dextro isomer with NMDA antagonist activity (114).
Agonist–antagonist—or kappa agonist—opioids, including pentazocine, nalbuphine, and butorphanol, have predominantly kappa agonist effects while antagonizing the mu receptor. These drugs have been widely regarded as having less potential for abuse and addiction than the pure mu agonists, though elective use for reward and the development of addiction to these medications have been observed. The actions of kappa agonists are complex and may include analgesia as well as antagonism of the effects of rewarding drugs, which has made their study a focus for addiction treatment drug development (115). Their clinical usefulness as analgesics is limited by a number of factors. They exhibit a ceiling effect in terms of analgesia and cannot be titrated for severe pain. Their use sometimes is associated with dysphoric reactions. Because of their mu antagonist activity, they may reverse analgesia and precipitate withdrawal in individuals who are physically dependent on mu agonists. Consequently, no clear advantages of agonist– antagonist drugs have been demonstrated in the treatment of pain, though they may be a reasonable choice in some patients. There is some evidence that kappa agonists may have greater analgesic efficacy in women (116).
Partial mu agonists, including buprenorphine and tramadol, provide analgesia via mu opioid receptors but have relatively low intrinsic efficacy; that is, they bind to mu opioid receptors but produce less receptor activation than full mu agonists. Clinically, they are very different medications.
Buprenorphine is a partial agonist opioid with complex pharmacology (117) that is available in parenteral, sublingual, and transdermal preparations. It has a long half-life and high receptor affinity and is useful in treatment of opioid addiction (118). In the United States, sublingual or transmucosal buprenorphine is FDA approved for treatment of addiction by clinicians with waivers from the Centers for Substance Abuse Treatment to provide this treatment. As an analgesic, the transmucosal/sublingual form can be used off label at 6-to 8-hour intervals for moderate to moderately severe pain (119). Buprenorphine has traditionally been thought to have a ceiling effect as an analgesic and, therefore, not able to provide continuously increasing analgesia beyond a certain dose titration; however, this belief has been challenged (120), and there is no clear consensus on titration. Buprenorphine is also available in the United States in transdermal form with an indication for pain management (121). While buprenorphine’s abuse potential has been thought to be relatively low, it appears to in fact have significant diversion potential. The reasons for this are complex and may include both use for reward, particularly in combination with other drugs, but more commonly use to prevent withdrawal when other opioids are not available for use (122).
Tramadol, which generally is used in oral form, has a second mechanism of analgesia through the inhibition of reuptake of serotonin and norepinephrine. Doses are limited by a significant potential for seizures at levels above 400 mg/d, which thus is its effective analgesic ceiling. Tramadol appears to have less abuse potential than pure mu opioid analgesics, though there are reports of abuse and addiction (123).
Routes of Administration
Opioids may be administered orally, rectally, transmucosally, nasally, intravenously, subcutaneously, transdermally, and intraspinally. The oral, enteral, or transdermal routes generally are preferred when feasible because they are not invasive and usually provide satisfactory analgesia, even when high doses are required. However, when these routes are not reasonable (as when patients are unable to take medications orally or when rapid titration is necessary), parenteral routes may be preferred. Note that parenteral routes increase both speed of onset and peak blood levels obtained, which may be favorable in terms of analgesia but also may increase reward, a consideration in persons with SUDs, and may increase sedation and other side effects.
IV access may be difficult in individuals with a history of injection drug use; for such patients, surgical venous access may be necessary, or continuous subcutaneous infusions may be used. Intramuscular injections are effective, but discouraged because of the pain involved in repeated injections and the variable blood levels obtained. If side effects of systemic use are not acceptable, intraspinal opioids may be indicated. Epidural administration is commonly used postoperatively, when a catheter may already be in place. For longer-term use, intrathecal analgesia is more commonly provided. Rectal preparations may be useful for patients who are vomiting or who are unable to take oral medications. Sublingual or transmucosal administration of some preparations is clinically effective as well; these have a rapidity of onset and peak levels generally intermediate to oral and IV use.
Dose Titration and Scheduling
Measuring Efficacy
The serial use of a pain scale, such as a numerical 0 to 10 rating scale or a visual faces scale, is helpful in assessing response to pain treatment (124). In treating chronic pain, it is often helpful to inquire about worst pain, best pain, and typical pain experienced in a particular period of time, such as the past week or month, and to document what is an acceptable level of pain that permits satisfactory comfort for the individual as well as function and quality of life. Pain ratings may be affected by myriad co-occurring issues and experiences, so the target numbers viewed as indication of satisfactory treatment must be individualized. In patients who cannot use a numerical rating scale, verbal descriptors such as none, mild, moderate, severe, and excruciating may be preferred. In the context of chronic pain treatment, assessment of function may have equal or greater importance than measurement of pain per se (as discussed in section on Treatment of Chronic Non-Terminal Pain later in this Chapter and in Chapter 95 Rehabilitation Approaches to Pain Treatment).
Dose Requirements
Several factors must be considered in determining the dose and interval of administration that will provide effective analgesia in a given patient for a given problem. The pharmacokinetics and potencies of the drugs must be taken into consideration, along with idiosyncratic patient responses to different opioids (104,105,229). Finally, the tolerance of individuals who have been exposed to opioids on a prolonged basis (whether therapeutically or due to addiction) must be accommodated (40). Individuals who are currently using opioids or have used them persistently in the past are likely to be relatively tolerant or may develop rapid tolerance to the analgesic effects of opioids and, therefore, may require relatively high doses at relatively short intervals to achieve analgesia. If the patient used opioids on a daily basis prior to the onset of acute pain, then his or her usual dose of opioids cannot be expected to provide analgesia for acute pain, and additional treatment must be provided.
Long- versus Short-Acting Medications
Long-acting medications include those that are intrinsically long acting, such as methadone, levorphanol, and buprenorphine, and medications that are long acting by virtue of being formulated for slow release, such as controlled-release and transdermal preparations. Most other opioids are shorter acting and immediate release.
When patients have persistent pain on an around-the-clock basis, longer-acting medications offer several advantages. They provide relatively stable drug blood levels and, therefore, more consistent analgesia than frequent doses of short-acting medications. Fewer peaks and valleys may result in fewer side effects, including less reward. When longer-acting medications are taken on a scheduled basis, patients are less driven to focus on pain and medication use because there is usually less opportunity for pain to break through and less need to watch the clock to note when medication is due.
Despite these theoretical advantages of long-acting or sustained-release opioids, few studies have directly compared analgesia, side effects, reward, or misuse in persons prescribed different specific opioids or formulations of opioids. One such study found no significant difference in analgesia or misuse between short-acting hydrocodone and longer-acting methadone (125). In addition, it is unclear whether round the clock or intermittent dosing is more likely to drive tolerance, hyperalgesia and/or dependence. Any theoretical advantages of long-acting opioids, therefore, must be weighed against other clinical issues.
It is important to be aware that most controlled-release opioids can be altered to become immediate-release drugs through chewing, crushing, snorting, or extracting and injecting. Many persons who use prescription opioids to get high do in fact alter them in some way (70,126). Currently, a number of abuse-deterrent formulations are available, and the FDA has released guidance to industry on the development of future abuse-deterrent medications (127), though it is not likely that any system will be entirely abuse proof. Strategies under development to reduce potential for abuse include embedding the opioid with an antagonist (naltrex-one) or aversive substance (such as ipecac or capsaicin) that are released with tampering and creating a chemical matrix from which the opioid cannot easily be released with chewing, crushing, or chemical extraction (128,129).
Frequent use of short-acting medications may result in increased pain due to “on–off effects” as blood levels fluctuate between peak and trough, resulting in alternating analgesia and incipient withdrawal in persons with physical dependency. Withdrawal-mediated pain appears to be in part due to hyperexcitability of mu receptor containing neurons through up-regulation of cAMPs increasing spinal pain transmission (130). In addition, increased cellular activity in the rostral ventromedial medulla may also contribute (131). Sympathetic arousal and muscular tension associated with withdrawal may also play a role in increased pain. Such rebound phenomena can theoretically be avoided by carefully scheduling doses to maintain fairly constant blood levels, but stable blood levels are easier to achieve with longer-acting medications.
Scheduled versus As-Needed Opioids (PRN)
Shorter-acting medications are often helpful for intermittent pain, for exacerbation of low-grade pain, or for evoked increase of pain treated with around-the-clock opioids. There are several issues to consider in scheduling opioids, particularly in individuals with, or at risk for, substance misuse. First, pairing the perception of pain with the administration of a rewarding, and therefore potentially reinforcing, drug can theoretically reinforce the perception of pain and lead to increased use of the drug and increasing distress (132,133). Such potential reinforcement of pain may be modified by making opioid use time contingent or activity contingent rather than contingent on the experience of pain. In time-contingent dosing, a patient who routinely develops pain in the afternoon and evening, for example, might receive a routine dose of short-acting opioid at noon and 5 PM only. In activity-contingent dosing, someone who has unmanageable pain in association with certain valued activities, such as sitting on hard wooden pews, might be instructed to take medication 30 minutes before the activity. In addition to reducing reinforcement of pain and medication taking, such strategies may improve pain control by preempting it.
Scheduling opioid doses in supervised care settings, such as hospitals or nursing homes, also eliminates the need for patients to request medications, which can result in delayed treatment and create a perception that the patient is “constantly seeking opioids.” When opioids are scheduled, the patient may decline doses if pain is well controlled.
For constant pain at a moderate level of intensity, when an opioid is indicated, a relatively low dose of a long-acting pure mu agonist (such as methadone or a controlled-release preparation of morphine, oxycodone, or other opioid) or a combination product containing an opioid and acetaminophen or an NSAID may be appropriate. For severe pain, a pure mu agonist is usually indicated unless a neural blocking procedure or other intervention controls the pain. That should be a drug without a ceiling effect, rather than a mixed agonist–antagonist or product containing acetaminophen or salicylate (which can be toxic with titration) and should not have toxic metabolites (such as meperidine).
If pain is continuous, PCA, a continuous parenteral infusion, or long-acting oral or transdermal medications, depending on the context, are appropriate. In acute or cancer pain, rescue doses of PRN medications are usually provided for breakthrough pain or exacerbations of baseline pain. The frequency and doses of rescue doses must be determined according to the context (134). When multiple doses are required each day, this generally indicates a need for a higher baseline dose of long-acting medications.
In the chronic nonterminal pain setting, when long-acting opioids are used for continuous baseline pain, some clinicians prefer that patients manage the daily ups and downs of pain with activity pacing and use of nonopioid interventions (heat, ice, stretch, relaxation, etc.) to prevent or address mild exacerbations, rather than using frequent doses of PRN medications. Some provide a few PRN doses per month or per week for more major exacerbations of pain to deter unnecessary frequent ER or clinic visits. However, some chronic pain patients have predictable activity-related pain that may require regular short-acting doses in association with the activity.
Patient-Controlled Analgesia
PCA can be used successfully for management of acute pain in individuals with addictive disease and sometimes is the preferred method of providing postoperative and posttraumatic pain control. It often is used in the setting of advanced cancer pain as well. PCA allows the patient to self-administer small incremental doses of opioids intravenously or subcutaneously and thus provides stable analgesic blood levels. It usually produces more uniform pain relief at a lower total dose of medications than bolus dosing or continuous infusions (44). It avoids peaks (which may cause sedation or intoxication) and valleys (which may result in pain, anxiety, and drug craving). As with scheduled dosing, the use of PCA eliminates the need for the patient to request opioids and thus avoids potential conflicts between patients and staff, which can arise when persons with addictive disease request opioids (135). For persons with significant opioid tolerance, a background infusion may be used with PCA.
Because PCA requires self-administration, it may create ambivalence in recovering persons and in patients with active addiction who have difficulty limiting their administration of opioids to levels that provide analgesia without intoxication. The latter problem may be managed to some degree through the physician’s control of the incremental dose size and frequency and the total dose available over a period of time. In theory, PCA may reinforce pain through the pairing of pain with self-administration of opioids and thus may make cessation of analgesic doses of opioids difficult. In practice, however, these issues rarely arise, probably because the small incremental doses provided by PCA provide relatively little reward.
Enhancing Compliance and Control
When scheduled medications are required on an outpatient basis in individuals with SUDs, it is helpful to give specific times for drug administration (e.g., “q 8 hours at 7:00 AM, 3:00 PM, and 11:00 PM”), rather than indicating that a drug should be taken three times a day or every 8 hours. This reduces the potential for confusion over dosing and possible resulting misuse.
If an individual has difficulty controlling medication use but needs opioids for pain relief, it may be helpful to have a trusted other, such as a partner or friend, dispense the medications, either by the dose or at time-limited intervals such as every 1 or 2 days. Daily or other short-interval dispensing also may be arranged through a visiting nurse, pharmacy, or hospice.
Opioid Side Effects and Management
Opioid side effects can be considered in two groups: side effects of acute and long-term opioid use.
Acute Use Side Effects
Common physical side effects of opioid use include respiratory depression, constipation, nausea and vomiting, urinary retention, and pruritus. Respiratory depression deserves special consideration because it can be life threatening.
Respiratory Depression
Respiratory depression is a potentially fatal side effect of opioid administration and demands awareness throughout treatment, particularly when opioids are first introduced or when doses are increased or specific opioids changed. Opioid-induced respiratory depression results from depression of brainstem respiratory responses to carbon dioxide (CO2) (136). Though CO2 response decreases in a dose-dependent manner with the administration of mu agonists, significant respiratory depression does not usually occur in the course of treating healthy patients with standard doses. Respiratory depression may be significant; however, when higher-dose opioids are used for acute pain in opioid-naïve patients, particularly those who are elderly or debilitated or in patients with obstructive or central sleep apnea who rely on CO2 response to awaken them during sleep if hypoventilation occurs or in patients who are also using sedative–hypnotics (137). Use of continuous positive airway pressure (CPAP) during sleep by patients with obstructive sleep apnea is prudent if they are to use opioids.
Significant sedation often is a precursor to respiratory depression and signals a need to hold medication and adjust the dose. Respiratory depression rarely is a problem in chronic opioid administration in healthy individuals if doses are increased gradually in response to analgesic tolerance, because tolerance to respiratory depression tends to occur more rapidly than to analgesia. Patients should be closely observed, however, when doses are abruptly increased or when patients are rotated from one opioid to another or in persons at risk for overuse. Special care also should be exercised in titrating opioids with long half-lives, such as methadone or levorphanol, because delayed respiratory depression may occur. Withholding of opioids and respiratory support are the first-line treatments for this side effect with reduction of a lower dose of opioid when appropriate. An opioid antagonist such as naloxone should be used in emergency situations with awareness that it may precipitate an acute abstinence syndrome in persons with physical dependence.
Pain acts as a stimulant for wakefulness and ventilation, so care should be taken when a patient using opioids for pain control undergoes a definitive procedure that alleviates pain, such as a nerve block or spinal cord ablation; in the sudden absence of pain, preprocedure doses of opioids may result in sudden sedation or hypoventilation. Doses should be reduced as indicated in response to reduced pain.
Other Acute Side Effects and Management
Side effects sometimes are specific to a particular drug in a particular individual, and it is often appropriate to simply substitute a different opioid to avoid a side effect such as nausea or pruritus. With the exception of constipation, side effects usually are transient and may improve or resolve with continued use of opioids at a stable dose. Persistent side effects can often be eliminated through treatments such as stool softeners, antiemetics, or antihistamines.
Side effects correlate with blood levels and maybe are minimized by limiting peak levels. To accomplish this, scheduled doses of long-acting or controlled-release opioids may be considered when oral preparations are used. Continuous infusions or PCA achieves the same goal when parenteral administration is required.
Constipation is a persistent side effect that may not resolve without treatment. It is attributed to direct action on opioid receptors in the intestinal wall. This causes a decrease in intestinal motility and results in dehydration of stool. It generally is advisable, therefore, to give both a stool softener and a bowel stimulant to effectively manage constipation. When long-term and/or high-dose use of opioids is anticipated, introduction of such treatment on a preemptive basis is recommended (138).
Hepatic, renal, and other organ toxicity generally are not reported with opioids as they are with many nonopioid analgesics, such as acetaminophen and NSAIDs. However, close observation and dose adjustments may be appropriate in persons who have impaired hepatic or renal function, which may result in reduced drug clearance.
Central nervous system (CNS) side effects of opioids may include sedation, cognitive dysfunction, and affective changes. Sedation and mild cognitive changes are common when opioids are introduced or when the dose is increased, but they usually resolve once a stable therapeutic dose of opioid is achieved and sustained for a period of time (139). Occasionally, however, CNS effects may persist when high doses of opioids are required, particularly when long-acting medications such as methadone are used in elderly or frail patients (140). Like many other side effects, sedation and cognitive dysfunction may be managed or avoided by changing medications, by continuous administration of the minimum dose necessary to achieve analgesia, or by administration of a treatment medication. When significant persistent opioid-induced sedation occurs in cancer pain patients or patients with other severe intractable pain, stimulants such as methylphenidate and dextroamphetamine may be helpful (141). The use of stimulants, which may be abused by some individuals, requires the same caution in patients with addictive disorders as that required in the use of opioids.
Pain, particularly chronic pain, often is associated with negative mood states such as anxiety or depression. These frequently resolve with effective pain treatment. However, if depression, anxiety, dysphoria, or other distressing affective symptoms occur in the course of analgesic therapy, opioids should be reviewed as a possible contributing factor.
When opioids are necessary and side effects persist despite rotation and dose adjustment, pharmacologic management may be appropriate.
Long-Term Use Side Effects
Side effects of long-term opioid use may include hyperalgesia, respiratory depression, and constipation (all discussed earlier) as well as effects on the endocrine and immune systems.
Endocrine Effects
Opioids affect the hypothalamic–pituitary–adrenal and the hypothalamic–pituitary–gonadal axes. Effects in human and animal models include alterations in activity of growth hormone, prolactin, luteinizing hormone, testosterone, estradiol, oxytocin, thyroid-stimulating hormone, vasopressin, and adrenocorticotropic hormone (142). Interactions between opioids and endocrine systems are complex and vary with the type and duration of opioid used as well as the gender and medical comorbidities of users. However, clinically significant effects with prolonged use may include androgen deficiency and bone loss (143).
Androgen deficiency, including low testosterone levels in men, can manifest in depressive symptoms, fatigue, low libido, and erectile dysfunction (144). Many experts recommend routine testing of all men receiving long-term opioid therapy, and there is clear evidence of symptom improvement when symptomatic low testosterone levels are identified and treated (145,146). In women, gonadotropin dysfunction has been shown to include lowered levels of follicle-stimulating hormone, luteinizing hormone, estradiol, dehydroepiandrosterone, and testosterone with common clinical manifestations of oligomenorrhea or amenorrhea. Estrogen replacement therapy in consultation with an endocrinologist is sometimes appropriate (147,148).
Increased rates of osteopenia and osteoporosis have been observed in long-term opioid users and may be related to opioid-induced changes in bone metabolism and endocrine function (149,150). However, studies to date have been confounded by numerous variable including medications, activity levels, smoking, and others, so the mechanisms are not clear and it is also unclear whether bone density screening may be indicated for all persons receiving long-term opioids (151). The concern is of special significance, however, in older persons who may be at risk of falls and fractures related to the use of opioids (152).
Immune Effects
Effects of opioids on the immune system have been demonstrated, with numerous studies both in animals and humans suggesting immunosuppressive effects, particularly on cell-mediated immunity (153). However, no clear clinically significant effects of opioids on immune function have been demonstrated to date, and the clinical relevance of these findings is therefore uncertain (154). Because pain itself has been demonstrated to have some immunosuppressive effects (154a), the relative balance of these effects must be determined.
Because side effects often vary from one opioid to another in different patients, rotation from one opioid to another is sometimes helpful in resolving side effects that do not resolve over time or that do not respond to pharmacologic treatment.
Changing Opioids
Transition from one opioid or form of opioid to another may be indicated in a number of circumstances: when tolerance occurs to a specific opioid with loss of analgesic efficacy, when a patient on chronic oral opioids becomes NPO, or when significant side effects occur and persist. Opioid rotation may result in improved analgesia when tolerance is present, sometimes on significantly lower equivalent doses of the new opioid. Rotation back to the original opioid—or an alternative—can occur if tolerance develops to the new opioid (155). Opioid rotation has been found to be clinically useful in both cancer-related pain and chronic non–cancer-related pain (156,157).
Traditionally, opioid analgesic equivalency charts have been used to calculate equianalgesic doses, and the new opioid has been given at 50% to 75% of the calculated equi-analgesic dose to account for incomplete cross-tolerance (except when methadone is the new medication in which case the dose reduction was much lower––to 10% to 25% of the calculated dose or less). However, this practice has been called into question as potentially unsafe for several reasons: Equianalgesic studies are based on single-dose studies rather than chronic administration, equianalgesic charts vary significantly in dose equivalents listed, and charts cannot take into account interpatient variability in opioid responsiveness and tolerance (157,158). In addition, it has been observed that there is a risk of overdose when new opioids are started, suggesting the need for a new paradigm of rotation (159). It should be noted that some methadone conversion tables in use are clearly incorrect and suggest a 2:1 methadone to morphine potency ratio with oral morphine, which is only true at very low doses. At higher doses, the ratio is closer to 16:1. This, plus the extreme interpatient variability in methadone half-life, renders the use of any conversion ratio hazardous when rotating to methadone from other opioids. Each patient must be individually titrated, starting with quite low doses of methadone.
As an alternative, it is suggested that equianalgesic charts be used to guide starting doses and intervals of administration of the new opioid and the new opioid should be given within its usual range of effective starting doses and titrated gradually with careful observation (159). If the patient is reliable in managing their opioid medication, PRN doses of short-acting opioid can be provided and used if analgesia is inadequate and there is no sedation present. It is critical to be aware of the potential of delayed respiratory depression, particularly with drugs with long half-lives such as methadone and to monitor carefully and instruct a patient to hold medication for sedation (which most often precedes respiratory depression).
Sometimes, the transition from one opioid to another is better tolerated if the patient is gradually “rolled over” from one medication to another. That is, the old medication is tapered while the new medication is increased incrementally over a few days or weeks. This permits observation of the patient’s response to the new drug and adjustment of the dose to avoid side effects and maintain analgesia.
Management of Opioid Withdrawal
In the acute pain setting, most patients taper their medications without incident as pain gradually improves. However, tapering sometimes is impeded by fear, withdrawal, or craving. The goal of tapering is to provide stable but decreasing blood levels of opioid so as to prevent precipitous troughs. Though stable blood levels usually are only approximated with the use of short-acting medications, most patients do not experience significant withdrawal while gently tapering in the acute pain context.
If the patient has been using an intermittently administered medication such as bolus parenteral morphine or oral oxycodone, the interval of administration can be decreased somewhat as the dose is decreased in order to avoid low blood levels between doses. Alternatively, the patient can be transferred to a continuous infusion or a longer-acting oral medication (such as methadone, controlled-release morphine, or oxycodone) and the dose gradually reduced. Some patients may benefit from having their medications dispensed daily or dose by dose while tapering in order to assist in controlling use as the dose is decreasing.
If abrupt cessation of opioids is necessary, an acute withdrawal syndrome may be attenuated to some degree through the prescription of alternative medications. An alpha-2-adrenergic drug such as clonidine may be helpful in attenuating autonomic signs and symptoms of withdrawal (160,161), while NSAIDs or acetaminophen can relieve muscular aching. A gastrointestinal antispasmodic such as dicyclomine may relieve gastric cramping distress. A benzodiazepine or other sedative–hypnotic may be used on a short-term basis to reduce irritability, anxiety, and sleeplessness. Alternatively, sedating antidepressants such as trazodone, doxepin, or gab-apentin have been demonstrated to have similar effects.
Withdrawal of chronic opioid analgesics can be managed similarly; however, more gradual withdrawal using methadone or buprenorphine or an alternative long acting is often particularly helpful in this context (162,163). Protocols for management differ widely, but many patients tolerate a 10% reduction in opioid dosing every 1 to 2 days, with more gradual reduction toward the end of tapering. It is important to note that withdrawal of opioids used for pain can be legally supervised by any physician with a DEA license; however, detoxification from opioids as a component of addiction treatment in an opioid-addicted person can only be done with buprenorphine by a waivered buprenorphine provider and with methadone by a licensed treatment center.
PAIN MANAGEMENT IN PERSONS WITH ADDICTION
Acute Pain
Inadequate treatment of pain increases morbidity after trauma and surgery (164), whereas optimal pain treatment appears to shorten hospitalizations in similar contexts (165). Pain increases distress and anxiety and may become a significant risk factor for relapse in those in recovery and for aberrant use in those with active abuse or addiction. Individuals with addictive disease often experience particularly high levels of anxiety in association with trauma, illness, or surgery, because they fear that their pain will not be adequately managed. This, in turn, can affect how they experience and react to pain. Attention to their concerns may facilitate pain management.
Principles of care that may facilitate management of the patient with pain and concurrent SUD include addressing the addiction directly, identification and treatment of withdrawal when present, provision of effective treatment of pain, accommodation of tolerance when present, and clear documentation of the treatment plan.
Address the Addiction Disorder
An open and nonjudgmental approach to the discussion of substance use concerns may help to facilitate information exchange with patients regarding their use. Often, health professionals discuss concerns about a patient’s substance use among themselves, without bringing the patient into the discussion. When addiction is understood as a medical disorder, it becomes easier to address it in the same manner as any other medical condition, with respectful, but matter-of-fact, concern. Patients with addiction disorders often justifiably fear that awareness of their problem will negatively affect the manner in which their physicians and other providers approach their care (166). Therefore, they may not be immediately forthcoming about their addiction. It is helpful to allay the anxiety by reassuring the patient that the addiction will not impede efforts to treat their pain. For recovering patients, reassurance that effective acute pain management is not likely to lead to relapse is also helpful.
The patient who is hospitalized for an acute medical problem may be more open to intervention for his or her addiction disorder than he or she would be as an outpatient (167). It is important to capture such windows of opportunity to help bring patients into recovery. Addiction treatment should be offered when it is detected in the course of pain treatment. Counseling may be initiated at any time, as long as acute pain is adequately controlled. If the patient does not accept addiction treatment at the time it is offered, it may be helpful to use the acute pain problem to begin to explore the patient’s motivation for recovery and to followup at future visits. Recovering persons often benefit from increasing their recovery activities during times of stress, such as hospitalization, trauma, and pain.
Many clinicians and individuals in recovery believe exposure to opioids, sedative–hypnotics, or anesthetics—even if not the patient’s drugs of choice—may lead to relapse. However, the distress of inadequately treated physical pain may also pose a risk of relapse (168). Effective pain treatment by whatever means, coupled with an active addiction recovery program, probably is the best support for continued recovery during periods of acute stress, including periods of pain.
Prevent or Treat Withdrawal
If a patient is dependent on opioids, it is usually necessary to continue opioids, even if the acute pain situation is resolved or effectively managed through an alternative approach, such as an epidural catheter or nerve block. If a nonopioid treatment is selected by the patient and physician, opioids can be either tapered gradually to prevent withdrawal or continued at a dose that avoids withdrawal but does not oversedate the patient. Abrupt cessation of opioids will usually cause acute increase in pain due to withdrawal.
It is permissible under the U.S. Controlled Substances Act for a treating physician to provide opioids to prevent withdrawal in a patient who is hospitalized for a diagnosis other than addiction. For example, if a patient with heroin addiction is hospitalized with multiple fractures after a motor vehicle crash or with subacute bacterial endocarditis related to heroin use, the treating physician can legally provide opioid medications, including methadone, to prevent withdrawal, as well as additional medications for pain. Though opioids for pain treatment can be continued after discharge, opioids cannot be provided for treatment of addiction after discharge, except from a licensed addiction treatment program (methadone treatment) or from a certified buprenorphine provider (169).
A patient dependent on alcohol or nonopioid drugs should have withdrawal symptoms treated when they occur in the course of pain treatment. Unrecognized alcohol withdrawal will make pain control difficult to achieve, and physical signs of withdrawal (such as hypertension and tachycardia) may be misinterpreted as reflecting acute pain. Usually, a long-acting benzodiazepine such as oral chlordiazepoxide or diazepam if the patient is able to take oral medications is an appropriate choice, though other benzodi-azepines such as lorazepam or oxazepam may be selected if parental use is required or if hepatic dysfunction is present.
Include the Patient in Clinical Decision Making
It is often helpful to include the individual with pain in the decision-making process regarding medication choices, dosing, and scheduling. This provides a sense of control and allays anxiety. It also may afford information that is useful in designing an effective treatment regimen. Addicted patients and patients with therapeutic drug dependence often know the doses they require to meet their basic dependence needs as well as the additional levels required to treat their acute pain. Such consultation may at times lead to a request for a dose beyond that needed for analgesia, so prudence in prescribing is required. If a patient becomes intoxicated or sedated at the prescribed dose, medications can be adjusted to avoid the observed side effects while continuing to provide analgesia.
Provide Effective Pain Relief
Pain relief should be provided in an effective and timely manner. Without adequate control of acute pain, it is unlikely that the patient will be able to engage in addiction treatment. Undertreatment of pain also may create longing for pain-relieving medications as well as anxiety, frustration, anger, and other feelings that tend to feed addiction (170). When they are effective, readily available, and safe, nonpharmacologic treatments—such as cold, transcutaneous electrical nerve stimulation (TENS), or regional anesthesia—are preferred by some clinicians and patients over systemic medications for the treatment of acute pain in individuals with addictive disease. When medications are indicated to relieve pain, those that are the least likely to alter mood may be used, if they are effective. Though exposure to dependence or reward-producing drugs may be one component of the development of addiction or relapse to drug use, such exposure alone does not necessarily create addiction or trigger relapse. When opioids or other such drugs are needed to manage pain, they should be provided at effective doses.
It is important to achieve pain relief with methods that do not confuse, stress, or frustrate the staff or the patient. For example, an epidural anesthetic may be ideal for post-thoracotomy pain in patient with an active or prior addiction disorder, but if hospital staff are inadequately trained in epidural catheter management, other options are preferable. Patients with acute pain should be monitored at regular intervals to assess the effectiveness of analgesia and the presence of side effects. Most patients will naturally taper their medications as acute pain resolves. Some patients with addiction disorders, however, benefit from the negotiation of a structured tapering of medications on a scheduled basis. Persistent use of opioids despite apparent healing is discussed below.
Accommodate Preexisting Opioid Dependence
Individuals who are physically dependent on opioids prescribed for pain or for addiction or who are dependent on illicit opioids must have their baseline opioid requirements met in addition to receiving additional opioids for analgesia. Chronically administered opioids will not usually address additional acute pain (171). The average baseline daily dose of opioid should be determined or estimated and either the same drug provided at that dose or an alternative opioid provided at a significantly lower than calculated equianalgesic dose (see section on Changing Opioids in this chapter).
Patients on Methadone Maintenance Therapy of Addiction
Methadone provided for addiction should usually be continued and supplemented with a different opioid for acute pain, rather than being entirely switched to an alternative opioid or having the methadone increased for pain. This is recommended for several reasons. First, incomplete cross-tolerance of methadone with other mu agonists has been noted and methadone withdrawal has been observed in some patients despite calculated equianalgesic doses of alternative mu agonists. Second, though an increase in methadone may be effective for pain management, its long half-life makes it difficult to safely titrate for acute pain. Finally, the use of the same drug for addiction maintenance and for acute pain management may confuse the issues of pain treatment and addiction treatment when the acute pain resolves, and it is time to taper the analgesic. That said in experienced hands, titration of methadone for acute pain can be effective.
When possible, it is helpful to confirm patients’ daily dose of methadone with their methadone treatment program. If the dose cannot be confirmed, it is safest initially to give the patients reported dose in three or four divided doses of no greater than 30 mg, particularly if it is a high dose, so that doses can be modified according to patient response. If a patient cannot take oral medications, methadone can be given parenterally at half the documented oral dose. Liquid methadone “microenemas” have also been given via tuberculin syringe (no needle) in cancer pain with good results.
Patients Taking Buprenorphine for Pain or Addiction
Strategies for managing acute pain in individuals taking buprenorphine are emerging as experience accumulates. Buprenorphine binds avidly to opioid receptors and thus tends to block the action of other opioids; thus, it is sometimes challenging to obtain analgesia by adding another opioid (172). In addition, buprenorphine has kappa opioid receptor antagonist activity that may impact the actions of other opioids (173,174).
If acute pain occurs due to trauma or illness in a patient on buprenorphine, mu opioids can usually be titrated to higher doses to overcome the buprenorphine blockade. Use of an opioid such as IV fentanyl or IV or oral hydromorphone, which bind with relatively high affinity to mu opioid receptors, is often recommended, though morphine and others have been used effectively (175). Opioid titration in this setting should be done by an experienced clinician with close monitoring of the patient with nalox-one available. When acute pain can be predicted, such as after elective surgery, in persons on buprenorphine, some experts discontinue buprenorphine a few days before surgery (176,177). Carefully dosed methadone can be added if needed if withdrawal symptoms or craving emerge after stopping buprenorphine while waiting for surgery. However, other experts note the challenges of reinducing patients on buprenorphine once acute pain is resolved and report good results with continuation of buprenorphine and titration of an alternative opioid to achieve analgesia in the same manner as for unanticipated acute pain (178).
Alternatively, a patient on a low-dose buprenorphine (2 to 8 mg/d) may receive acceptable analgesia from a temporarily increased dose. Buprenorphine has been demonstrated effective in patients not currently using opioids in controlling acute pain (179,180). Because of its partial agonist properties and its near full receptor occupancy at relatively low doses, buprenorphine has traditionally been thought to have a ceiling effect for analgesia; however, this view has been challenged by recent studies and observations (181,182). Full understanding of the analgesic properties of buprenorphine is still in evolution.
Document the Pain Treatment Plan
It is important to be clear in communicating the pain treatment plan to all staff involved in caring for a patient with addictive disorder. Stigma and misunderstanding are widespread among health care personnel, and these may lead to inadequate pain management when the primary treating clinician is not available and the plan is not clearly documented. In the absence of a clear and consistent structure, the patient’s behaviors may foster confusion of pain and addiction issues. Written documentation of the plan, displayed in a prominent and accessible location, is usually helpful.
When Pain Persists Beyond Apparent Healing
Many factors may lead a patient to complain of pain and to evidence a need for pain medications despite expected and apparent healing from surgery, trauma, illness, or other pain-provoking pathology. Continuing complaints may elicit concerns that the patient’s behaviors reflect SUD rather than pain. However, it is important to methodically consider other possible explanations before concluding that this is the case.
First, the patient may have an undetected physical problem, related either to the original painful problem or to a separate process. A thorough search for such a cause should be undertaken. The search should include a review of nociceptive causes of pain, such as an abscess or undetected fracture, as well as less common and often overlooked neurogenic causes of pain such as a neuritis, differentiation pain, central sensitization, or evolving sympathetically maintained pain. A surprising number of common surgical procedures have protracted pain as a frequent complication, probably due to such factors as central sensitization and nerve trauma. Thoracotomies, herniorrhaphies, and hip/ knee replacements, for example, may have a 40% to 60% rate of protracted pain complications.
Second, the patient may be physically dependent on opioids and may be experiencing pain related to withdrawal as the medication is discontinued. Withdrawal may mediate pain through a variety of mechanisms, including alterations in sympathetic arousal, changes in muscle tone, and alterations in opiate and other receptor function. Tapering of opioids to avoid withdrawal is described earlier. If increased discomfort occurs during the course of an appropriately crafted medication taper, the patient should be reexamined for an undetected physical origin of the pain. If none is found, the taper can be continued and nonopioid alternatives (such as TENS, NSAIDs, or nerve block therapy) provided. In most cases, the increased discomfort will be transient if withdrawal was the cause. If pain persists and no physical cause can be identified, treatment should be as for chronic pain.
The third reason that a patient may seek to continue opioids when the underlying pain condition has resolved is that the medications may be ameliorating nonpain symptoms, such as sleep disturbance, intrusive memories, anxiety, or depression. Opioids have been observed to improve mood in some patients in pain (183), but more specific treatments usually are indicated and may allow tapering of pain medications in patients whose physiologic pain driver has resolved.
If a physical cause of pain, withdrawal-associated discomfort, or self-medication is not present, addiction reassessment is needed. In a general hospital population, the latter reason is probably less common than the first three possibilities; however, it is likely relatively more common in the population treated by addiction medicine specialists.
If addiction is suspected, the patient should be observed for behaviors suggestive of addiction, including loss of control, continued use despite harm, and preoccupation with opioid use, with the understanding that any of these may be seen in patients with significant pain that is not effectively treated. The patient’s drug and alcohol history, as well as his or her family history of addiction, may add further useful information.
If the patient has previously been in recovery, reengagement in recovery activities is important. Any active, untreated addiction is an indication for evaluation by an addiction specialist if available. If an addiction disorder is diagnosed, the patient’s opioids can be tapered and alternative pain treatments implemented, while treatment for addiction is provided. Unless an underlying physical cause is identified, pain may resolve or improve after discontinuation of medications and treatment of addiction (37,184).
If opioid taper is impeded by persistent craving or unsanctioned use, opioid agonist therapy, with either buprenorphine/naloxone or methadone, should be considered. If a taper is not possible because of recrudescence of pain despite alternative therapies, continued opioid therapy, coupled with the necessary structure and support, may be selected as a component of chronic pain treatment. If both persistent pain and opioid addiction are present, sometimes combined opioid agonist therapy for addiction and non opioid therapies for pain with collaborative care between addiction treatment providers and pain providers can be effective (see section in this chapter on management of Chronic Nonterminal Pain).
A final consideration in the differential of complaints of persistent pain despite apparent healing is that the pain may be factitious. Occasional persons may feign pain in order to divert medications for sale, for personal use, or to share with others. Opioids must be discontinued if an individual is found to be diverting them, as misuse in this manner is both illegal and presents significant risk to the public health. In addition, a physician who knowingly provides opioids to a person who is diverting them may be liable to charges of drug trafficking.
If the cause of reported persistent pain cannot be identified and there is no evidence that the patient is seeking drugs for nonanalgesic purposes, the clinician has two choices. First, opioids can be continued while the clinician monitors the patient’s pain, side effects, and function and continues to screen for a cause of pain. If the patient’s quality of life is preserved or advanced without negative consequences, it may be that an undetected basis for the pain is present. The second choice is to taper opioids while alternative pain treatments are introduced. Because opioids may induce pain or hyperalgesia in some contexts (see Hyperalgesia), pain may improve on their elimination. Which choice is appropriate for a particular patient is a decision best made by the clinical care team, with the patient’s informed participation.
Progressive Cancer-Related or Terminal Pain
It has been estimated that pain is present in 24% to 60% of persons undergoing cancer treatment, 58% to 69% of persons with advanced cancer, and about 33% of cancer survivors (185). As cancer survival has markedly increased, this creates a new population of persons with cancer or cancer treatment–related pain that is not necessarily related to a terminal illness.
Cancer may occur more frequently in individuals with addiction disorders than in the general population (186), probably because of pathologic effects of many substances of abuse, including tobacco and alcohol, and the fact that nicotine dependence is more common in persons with other addictions than in the general population (187,188). Treatment of advanced cancer-related pain in the patient with addictive disease is usually similar to that in the person without addictive disease; the comfort of the patient should be the primary goal. Opioids generally should not be withheld when they are needed and effective because of concerns regarding addiction. However, if opioid-related problems are diminishing the patient’s quality of life, it can be necessary to maximize external controls (e.g., having medications dispensed daily by others) and to insist on concomitant addiction recovery work or to rely on nonopioid treatments (189,190).
The “therapeutic ladder” developed by the WHO is an accepted model for the treatment of cancer pain (Fig. 97-3) (191). Stage 1 of the ladder is for the management of mild pain and involves the use of nonopioid analgesics such as acetaminophen, aspirin, or NSAID as well as adjuvant medications such as tricyclics, anticonvulsants, and topical agents for pain and such associated symptoms such as insomnia, depression, and anxiety. Stage 2 of the WHO ladder addresses moderate pain. It involves use of a weak opioid preparation that combines opioids with acetaminophen or NSAID. Adjuvant medications for neuropathic pain and other symptoms should be used as indicated. For patients with continuous moderate pain, it is also reasonable to use a low dose of a long-acting opioid. This may avoid the intermittent peak effects of short-acting opioids that some experience as rewarding and which may be disconcerting to some patients in recovery. If these are selected in place of combination medications, additional acetaminophen or NSAIDs usually should be added for their complementary analgesic effect.
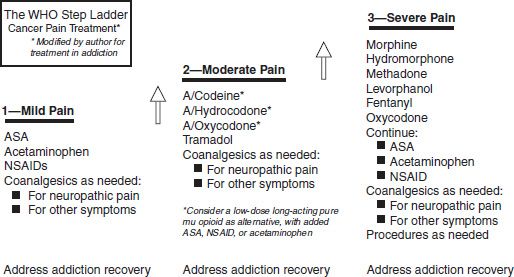
FIGURE 97-3 The WHO step ladder for cancer pain treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


