10
Opioid Pharmacology
This chapter will be most useful after having a basic understanding of the material in Chapter 18, Opioids, Analgesia, and Pain Management in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition includes:
• A history of analgesic use
• A detailed description of the endogenous opioid systems: ligands and receptors
• A discussion of opioid receptor classes, distribution, binding/coupling requirements for opiate ligands, and the functional consequences of acute and chronic opiate receptor activation
• Figure 18-8 Structures of morphine-related opiate agonists and antagonists
• Figure 18-9 Chemical structures of piperidine and phenylpiperidine analgesics
• Details of the cellular and molecular mechanisms of opioid tolerance, dependence, and withdrawal
• Nonanalgesic therapeutic uses of opioids
• Table 18-4 Resources for Pain Management
• Table 18-5 World Health Organization Analgesic Ladder
LEARNING OBJECTIVES
 Understand the mechanisms of action, adverse effects, and therapeutic uses of opiate receptor agonists.
Understand the mechanisms of action, adverse effects, and therapeutic uses of opiate receptor agonists.
 Know the mechanisms of action and therapeutic uses of opiate receptor antagonists.
Know the mechanisms of action and therapeutic uses of opiate receptor antagonists.
 Know the role of opiate receptor agonists in the management of acute and chronic pain.
Know the role of opiate receptor agonists in the management of acute and chronic pain.
 Know the different methods of administration of opiate receptor agonists that improve analgesic efficacy while reducing side effects.
Know the different methods of administration of opiate receptor agonists that improve analgesic efficacy while reducing side effects.
DRUGS INCLUDED IN THIS CHAPTER
• Alfentanil (ALFENTA)
• Benzonatate (TESSALON, others)
• Buprenorphine; injection (BUPRENEX); oral (SUBUTEX); in combination with naloxone (SUBOXONE)
• Butorphanol (STADOL, others)
• Codeine
• Dextromethorphan marketed for over-the-counter sales
• Difenoxin (MOTOFEN) available only in combination with atropine
• Diphenoxylate (LOMOTIL, others) available only in combination with atropine
• Fentanyl citrate (SUBLIMAZE, others); transdermal patch (DURAGESIC, others); fentanyl buccal tablets, buccal film, lollipop-like lozenges (FENTORA, ONSOLIS, ACTIQ, others)
• Hydrocodone (LORTAB, VICODIN, others)
• Hydromorphone (DILAUDID, others)
• Levorphanol (LEVO-DROMORAN)
• Loperamide (IMODIUM, others)
• Meperidine (pethidine, DEMEROL, others)
• Methadone (DOLOPHINE, others)
• Methylnaltrexone (RELISTOR)
• Morphine sulfate; liposomal formulation (DEPODUR); preservative-free for spinal delivery (DURAMORPH, DEPODUR, others)
• Nalbuphine (NUBAIN, others)
• Nalmefene—no longer marketed in the United States
• Nalorphine (NALLINE, others)
• Naloxone (NARCAN, others)
• Naltrexone (REVIA, VIVITROL, others)
• Naltrindole (δ receptor antagonist used only for biomedical research)
• Oxycodone (PERCODAN); in combination with acetaminophen (PERCOCET); extended release (OXYCONTIN)
• Oxymorphone (NUMORPHAN, others)
• Pentazocine (TALWIN); in combination with acetaminophen (TALCEN); in combination with naloxone (TALWIN NX)
• Propoxyphene (DARVON, others)
• Propoxyphene napsylate (DARVON-N)
• Remifentanil (ULTIVA)
• Sufentanil (SUFENTA, others)
• Tapentadol (NUCYNTA)
• Tramadol (ULTRAM)
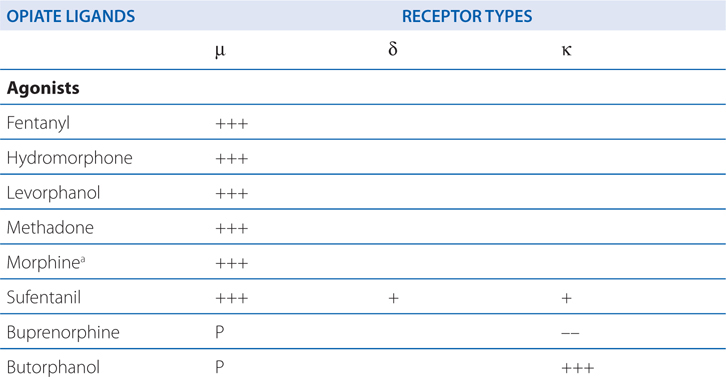
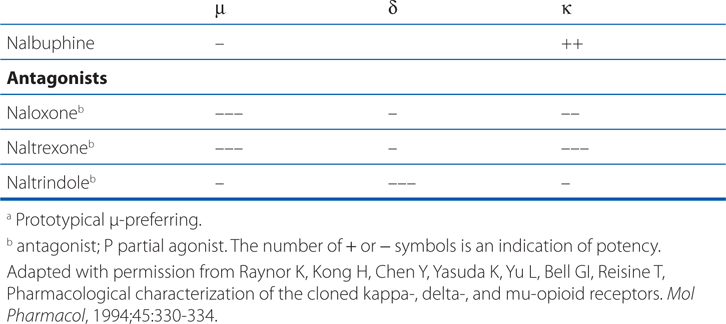
ACTIONS AND SELECTIVITIES OF SOME OPIOIDS AT μ, δ, κ RECEPTORS
MECHANISMS OF ACTION OF OPIOID AGONISTS AND ANTAGONISTS
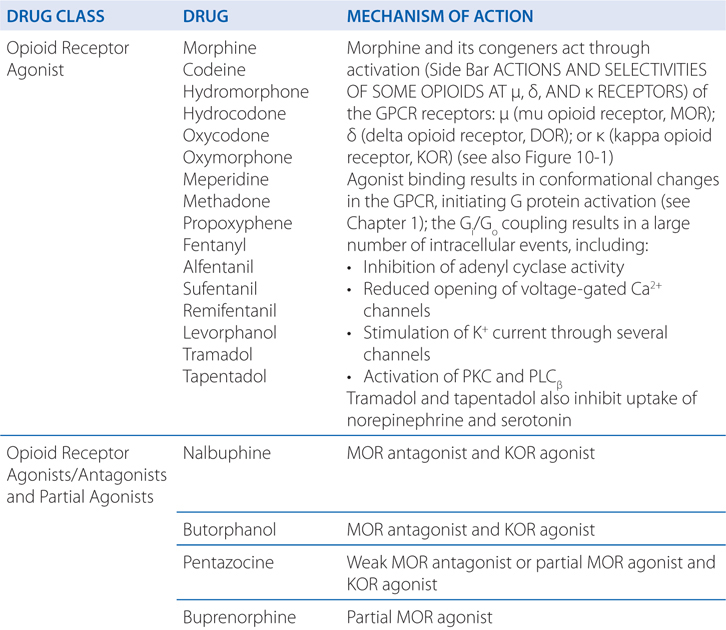
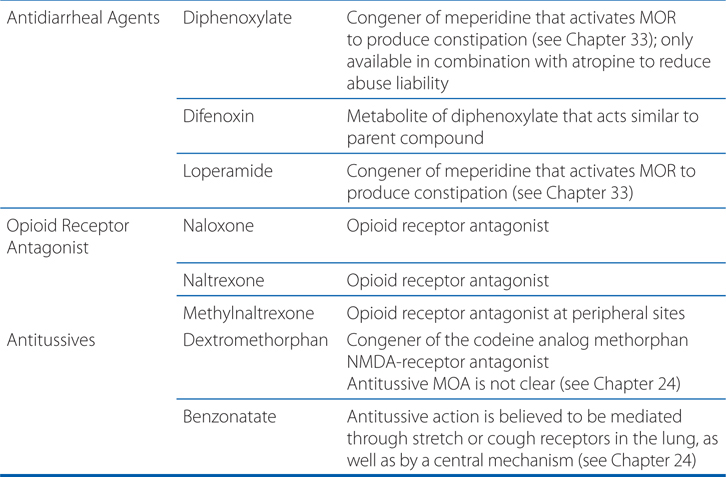
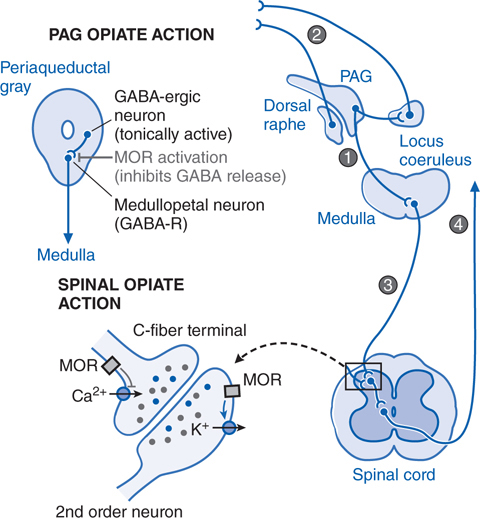
FIGURE 10-1 Mechanisms of opiate action in producing analgesia. Top left: Schematic of organization of opiate action in the periaqueductal gray (PAG). Top right: Opiate-sensitive pathways in PAG μ opiate actions block the release of GABA from tonically active systems that otherwise regulate the projections to the medulla (1) leading to an activation of PAG outflow resulting in activation of forebrain (2) and spinal (3) monoamine receptors that regulate spinal cord projections (4) which provide sensory input to higher centers and mood. Bottom left: Schematic of primary afferent synapse with second order dorsal horn spinal neuron, showing pre- and postsynaptic opiate receptors coupled to Ca2+ and K+ channels, respectively. Opiate receptor binding is highly expressed in the superficial spinal dorsal horn (substantia gelatinosa). These receptors are located presynaptically on the terminals of small primary afferents (C-fibers) and postsynaptially on second-order neurons. Presynaptically, activation of MOR blocks the opening of the voltage-sensitve Ca2+ channel, which otherwise initiates transmitter release. Postsynaptically, MOR activation enhances opening of K+ channels, leading to hyperpolarization. Thus, an opiate agonist acting at these sites jointly serves to attenuate the afferent-evoked excitation of the second-order neuron.
• Other medications
Other depressant medications such as alcohol, tranquilizers, sedative-hypnotics
• Sleep
Natural sleep produces a decrease in the sensitivity to CO2
Obstructive sleep apnea increases the likelihood of fatal respiratory depression
• Age
Newborns and elderly patients are at greater risk
• Disease
Patients with chronic cardiopulmonary or renal diseases can manifest a desensitization to increased CO2
• COPD
Enhanced respiratory depression is seen in patients with chronic obstructive pulmonary disease (COPD)
• Relief of pain
Removal of a painful condition (which can stimulate respiration) will reduce the ventilatory drive and lead to apparent respiratory depression
A 22-year-old man is brought to the emergency room following a skiing injury to his knee. Although he is in considerable pain, it is relieved by a small dose of morphine. He reports that his pain is still present, but it is less bothersome. He says the morphine “feels good.”
a. What are the mechanisms for the different causes of pain?
All pain is not the same and a number of variables contribute to the patient’s pain report and therefore to the effect of the analgesic. Many clinical pain syndromes, such as found in cancer, typically represent a combination of inflammatory and neuropathic mechanisms. The Side Bar PAIN STATES differentiates the various types of pain and Figures 10-2 and 10-3 show mechanistic diagrams of these types of pain. Although nociceptive pain (as in this patient) usually is responsive to opioid analgesics, neuropathic pain is typically considered to respond less well to opioid analgesics.
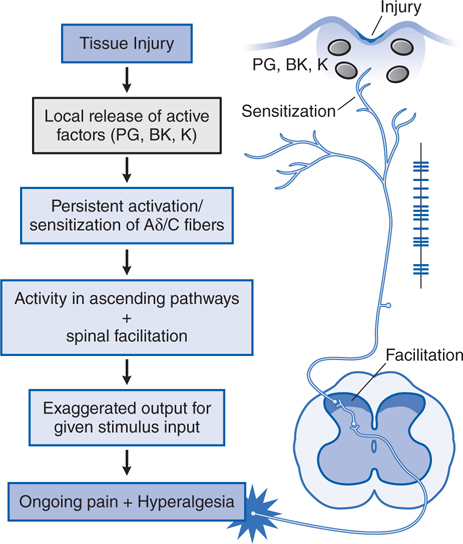
FIGURE 10-2 Mechanistic flow diagram of tissue injury–evoked nociception. BK, bradykinin; K, cytokines; PG, prostagladins.
Following tissue injury or local inflammation (eg, local skin burn, toothache, rheumatoid joint), an ongoing pain state arises that is characterized by burning, throbbing, or aching, and an abnormal pain response (hyperalgesia); such tissue injury-evoked pain is often referred to as “nociceptive” pain
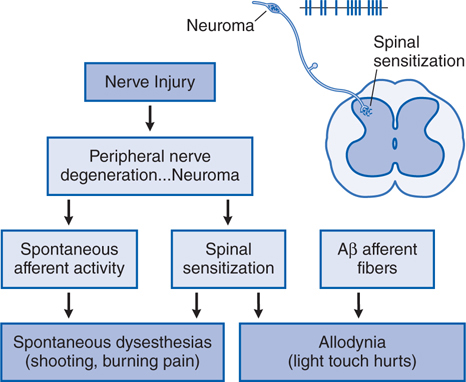
FIGURE 10-3 Mechanistic flow diagram of nerve injury–evoked nociception.
• Acute nociception
Acute activation of small high-threshold sensory afferents (Aδ and C fibers) generates transient input into the spinal cord, which in turn leads to activation of neurons that project contralateral to the thalamus and thence to the somatosensory cortex; examples include a hot coffee cup, a needle stick, or an incision
• Tissue injury (Figure 10-2)
• Nerve injury (Figure 10-3)
Injury to a peripheral nerve yields complex anatomical and biochemical changes in the nerve and spinal cord that induce spontaneous dysesthesias (shooting, burning pain) and allodynia (light touch hurts); this pain state is said to be neuropathic
b. What are the mechanisms of action for morphine and other opiate ligands to produce analgesia?
The analgesic actions of opiates after systemic delivery are believed to represent actions in the brain (supraspinal), spinal cord, and in some instances in the periphery. The supraspinal and spinal actions of opiates are shown schematically in Figure 10-1.
c. What are the mechanisms of action by which morphine and other opiate ligands alter mood and have rewarding properties that may be important in addiction?
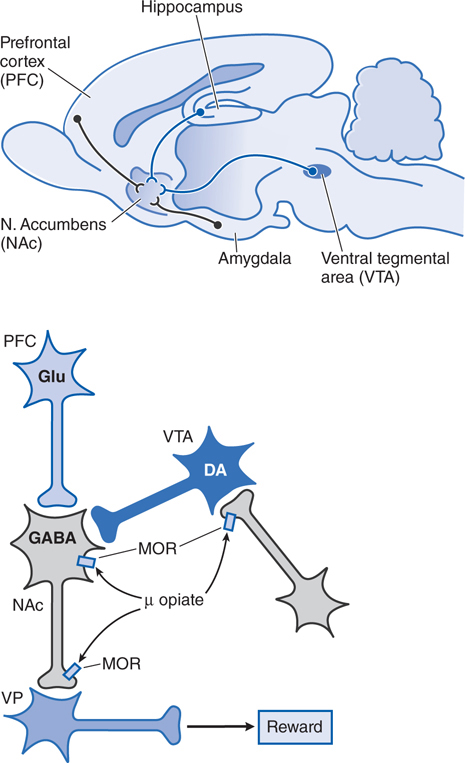
The mechanisms by which opioids produce euphoria, tranquility, and other alterations of mood (including rewarding properties) are complex and not entirely clear. Neural systems thought to mediate opioid reinforcement overlap with, but are distinct from, those involved in physical dependence and analgesia. Behavioral and pharmacological data point to a pivotal role of the mesocorticolimbic (MCL) dopamine system, a basal forebrain circuit long implicated in reward and motivation (see Figure 10-4).
FIGURE 10-4 Schematic pathways underlying rewarding properties of opiates. Upper panel: This sagittal section of rat brain displays simplified DA and GABA inputs from the ventral tegmental area (VTA) and prefrontal cortex (PFC), respectively, into the nucleus accumbens (NAc).
Following surgery for a hip replacement, a 64-year-old woman is treated with a parenteral opiate for pain. Upon release from hospital, she is given a prescription for oral oxycodone for pain. Three days after discharge she is complaining of constipation.
a. What is the cause of her constipation?
Opiates have important effects upon all aspects of GI function (see Chapter 33). It is estimated that 40 to 95% of patients treated with opioids develop constipation and that changes in bowel function can be demonstrated even with acute dosing. Opiate agonists suppress local neurogenic networks that provide a rhythmic inhibition of muscle tone leading to concurrent increases in basal tone in the circular muscle of the small and large intestine. This results in enhanced high-amplitude phasic contractions, which are nonpropulsatile. The upper part of the small intestine, particularly the duodenum, is affected more than the ileum. A period of relative atony may follow the hypertonicity. The reduced rate of passage of the intestinal contents, along with reduced intestinal secretion, leads to increased water absorption, increasing viscosity of the bowel contents, and constipation.
Lower panel: Neurons are labeled with their primary neurotransmitters. At a cellular level, MOR agonists reduce excitability and transmitter release at the sites indicated by inhibiting Ca2+ influx and enhancing K+ current (see Figure 10-1). Thus, opiate-induced inhibition in the VTA on GABA-ergic interneurons or in the NAc reduce GABA-mediated inhibition and increase outflow from the ventral pallidum (VP), which appears to correlate with a positive reinforcing state (enhanced reward).
b. What are the options for treatment of this patient?
The peripherally limited antagonists such as methylnaltrexone have a very important role in the management of the constipation and the reduced GI motility present in the patient undergoing chronic opioid therapy (as for chronic pain or methadone maintenance) and have been approved by the FDA for that use. With distribution restricted to the periphery, these agents do not alter central opioid agonist actions. Worrisome reports of GI perforation in this setting are under review by FDA. Other strategies for the management of opioid-induced constipation are described in Chapter 33.
A 43-year-old markedly obese man with the history of sleep apnea is being treated with hydrocodone for chronic back pain.
a. What considerations should be taken into account when treating chronic pain with opiates?
Management of pain is an important element in any therapeutic intervention. Failure to adequately manage pain can have important negative consequences on physiological function such as autonomic hyper-reactivity (increased blood pressure, heart rate, suppression of gastrointestinal motility, reduced secretions), reduced mobility leading to deconditioning, muscle wasting, joint stiffening, and decalcification, and can contribute to deleterious changes in the psychological state (depression, helplessness syndromes, anxiety). By many hospital-accrediting organizations, and by law in many states, appropriate pain assessment and adequate pain management are considered to be standard of care, with pain being considered the “fifth vital sign.”
Table 10-1 shows dosing data for clinically employed opioid analgesics. These are only guidelines, but they can give a place to start a patient’s treatment.
TABLE 10-1 Dosing Data for Clinically Employed Opioid Analgesics
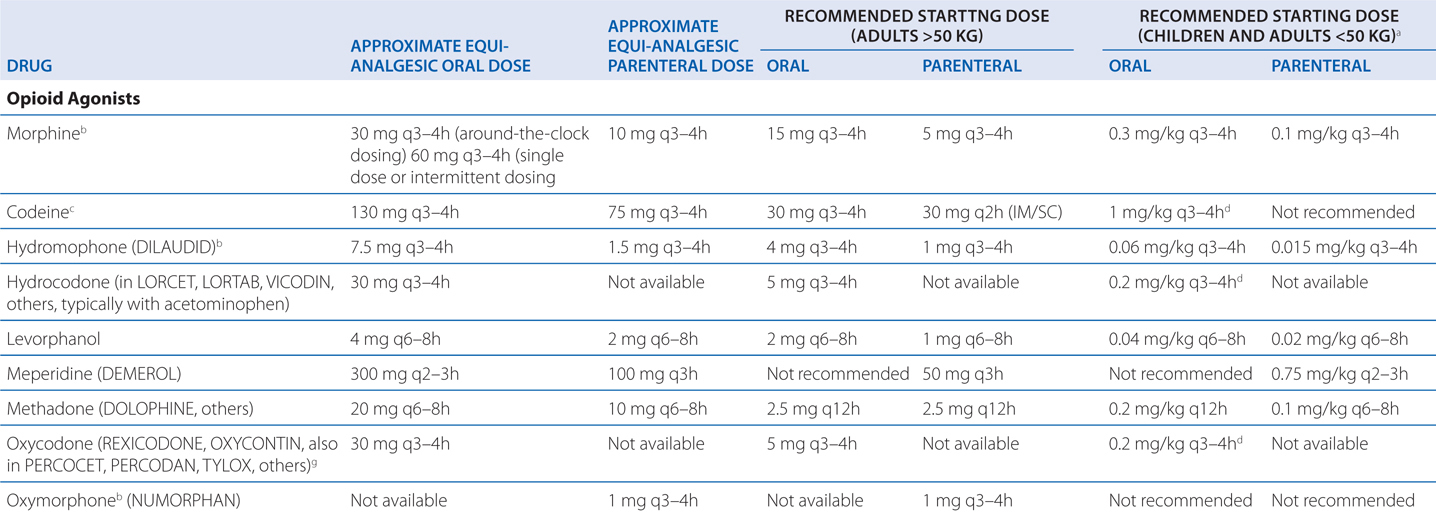
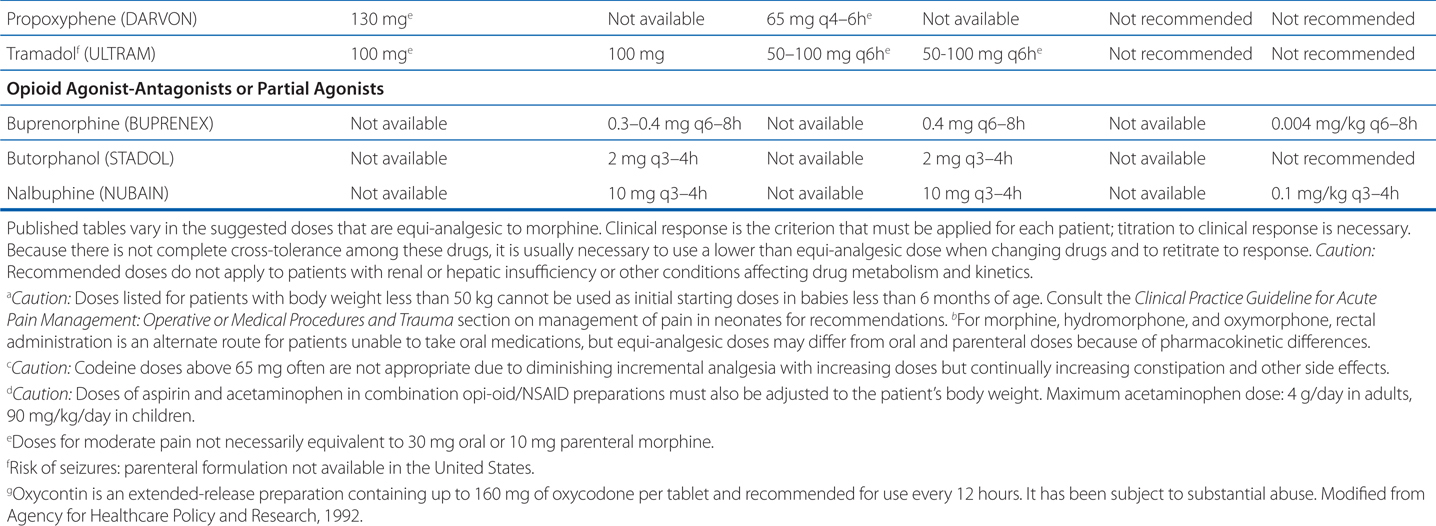
Numerous societies and agencies have published guidelines for the use of strong opioids in treating pain, including the American Academy of Pain Medicine, the American Pain Society, the Federation of State Medical Boards (FSMB), and the Drug Enforcement Agency. While slightly different in particulars, all guidelines to date share the criteria established by the FSMB (see Table 10-2).
TABLE 10-2 Guidelines for the Use of Opioids to Treat Chronic Pain
• Evaluation of the patient: A complete medical history and physical must be conducted and documented in the medical record.
• Treatment plan: The treatment plan should state objectives that are used to determine treatment success.
• Informed consent and agreement: The physician should discuss the risks, benefits, and alternatives to chronic opioid therapy with the patient. Many practitioners have developed an “opioid contract” that outlines the responsibilities of the physician and the patient for continued prescription of controlled substances.
• Periodic review: At reasonable intervals, the patient should be seen by the physician to review the course of treatment and document results of consultation, diagnostic, testing, laboratory results, and the success of treatment.
• Consultation: The physician should refer the patient for consultation when appropriate.
• Documentation/medical records: The physician should keep actual and complete medical records that include:
(a) medical history and physical examination; (b) diagnostic, therapeutic, and laboratory results; (c) evaluations and consultations; (d) treatment objectives; (e) discussion of risks and benefits; (f) treatment; (g) medications including date, type, dosage, and quantity prescribed; (h) instructions and agreements; and (i) periodic reviews.
• Compliance with controlled substances law and regulations: To prescribe, dispense or administer controlled substances, the physician must be licensed in the state and comply with applicable state and federal regulations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree