50
CHAPTER OUTLINE
■ UNIQUE ASPECTS OF OPIOID DEPENDENCE
■ CLINICAL ISSUES IN MAINTENANCE PHARMACOTHERAPY
■ MAINTENANCE TREATMENT USING METHADONE
■ MAINTENANCE TREATMENT USING BUPRENORPHINE
■ PREGNANCY AND OPIOID AGONIST TREATMENT
■ PATIENTS WITH CO-OCCURRING PSYCHIATRIC DISORDERS
■ GROWTH, CONTROVERSY, AND FUTURE CHALLENGES
Of an estimated 2 million opioid-dependent persons in the United States in 2010, approximately 330,000 are enrolled in programs offering opioid maintenance treatment (OMT), with 304,000 of those receiving treatment in 1,166 opioid treatment programs (OTPs) (1,2). Forty-five years of extensive research, clinical experience, and attention to public health concerns—combined with an extensive educational effort—have made OMT more available to those who need it. The 1,166 specially licensed OTPs in the United States offer counseling, testing, and maintenance pharmacotherapy using methadone (and buprenorphine in certain facilities), with daily dispensing and strictly regulated take-home medication for selected patients (2). Since 2002, office-based maintenance using sublingual buprenorphine has been available in the United States. A few OTPs also dispense buprenorphine as a second treatment option. This chapter focuses on maintenance pharmacotherapy in the context of the licensed OTP and is mostly about methadone maintenance. During the 1990s, several major scientific bodies examined the evidence regarding treatment benefits and access barriers; they concluded that OMT is effective and that barriers to obtaining it need to be reduced (3,4).
Since 1995, there has been an enormous upsurge in the nonmedical use of prescription opioids and sedatives. The National Survey on Drug Use and Health (NSDUH) reports that 1.9 million persons were regularly abusing or addicted to prescription opioids in 2010, with an additional 359,000 persons with heroin abuse or dependence (1). In many areas, nonmedical use of oxycodone and hydrocodone has overtaken heroin as drugs of abuse leading to OTP admissions. The parallel upsurge in nonmedical abuse of sedative medications is particularly dangerous for opioid-maintained patients, as the combination of benzodiazepines and opioids can be deadly.
Physicians are often unaware of the discrepancy between the benefits of maintenance treatment documented by scientific research and the public’s perceptions of such treatment; for this reason, it is necessary to begin with a description of the context in which OMT takes place.
HISTORY AND CONTEXT OF OMT
The modern use of opioids as a maintenance pharmacotherapy began with the use of methadone by Dole and Nyswander in the 1960s (5). Negative attitudes toward OMT have been common since that time among physicians, other treatment staff, patients, and the general public. These attitudes often stem from the perception that methadone treatment is “just substituting one addicting drug for another.” Rather than a simple substitution or replacement for illicit opioids, OMT involves a stabilization or correction of a possible lesion or defect in the endogenous opioid system (6,7). The neuro-biologic mechanism remains poorly understood. This intervention, by reducing opioid craving and preventing opioid withdrawal, reduces the likelihood of injection drug use, frees the patient from preoccupation with obtaining illicit opioids, and enhances the overall function, thus enabling the patient to make use of available psychosocial interventions.
Methadone maintenance has been shown to decrease mortality, reduce illicit drug use, reduce seroconversion to human immunodeficiency virus (HIV), reduce criminal activity, and increase engagement in socially productive activities (8–11). Nevertheless, a set of regulatory requirements unmatched by anything in medicine continues to contribute to the stigmatization of methadone as a treatment modality and creates many barriers to providing treatment to those who need it. Despite the 2001 reduction in regulatory barriers at the federal level (12), methadone continues to remain separate from the medical mainstream of care and be poorly understood by clinicians not involved in its daily application.
Negative attitudes affect OMT in a variety of ways (13). Physicians in other medical settings sometimes refuse to treat a patient who discloses that he or she is receiving maintenance pharmacotherapy. Occasionally, patients are told that they must withdraw from maintenance pharmacotherapy to receive treatment for other medical conditions. A physician may withhold medication needed for symptomatic relief, thus causing unnecessary discomfort and pain. Contrary to common beliefs, many opioid-dependent patients enter OMT with great ambivalence and want to discontinue maintenance therapy as soon as possible. Indeed, the initial hope of many practitioners, policy makers, and regulators was that methadone could be used to transition patients to a drug-free lifestyle and then be withdrawn. This has not proved to be the case. Early studies suggest that only 10% to 20% of patients who discontinue methadone are able to remain abstinent (14), a range consistent with clinical impressions and the findings of subsequent studies (10,15–17). This range is similar to that seen with many chronic medical conditions for which control requires the ongoing use of medication.
UNIQUE ASPECTS OF OPIOID DEPENDENCE
Although this chapter focuses on medical aspects of OMT, it is commonly accepted that addictive disorders are complex phenomena that involve the interaction of biologic, psychosocial, and cultural variables, all of which need to be addressed if the treatment is to be effective. As a medical modality based on proper use of opioid agonist medication, it should be clear that the medication itself is central to OMT as a treatment modality. Much of the destructive behavior of treatment professionals results from inappropriate expectations, particularly the belief that addicted persons could avoid drug use if they were sufficiently motivated. Vincent Dole, a pioneer in developing methadone treatment, always held the view that there is something unique about opioid addiction that makes it difficult for patients to remain free of illicit heroin use for extended periods of time. Prior to the discovery of the opioid receptor system, Dole and Nyswander (18) postulated the existence of a “metabolic disease,” a view supported and refined by subsequent biomedical research. Dole won the Albert Lasker Clinical Medicine Research Award in 1988 for his work in this area. He summarized his views in a paper in the Journal of the American Medical Association (7), in which he wrote the following:
It is postulated that the high rate of relapse of addicts after detoxification from heroin use is due to persistent derangement of the endogenous ligand narcotic receptor system and that methadone in an adequate daily dose compensates for this defect. Some patients with long histories of heroin use and subsequent rehabilitation on a maintenance program do well when the treatment is terminated. The majority, unfortunately, experience a return of symptoms after maintenance is stopped. The treatment, therefore, is corrective but not curative for severely addicted persons. A major challenge for future research is to identify the specific defect in receptor function and to repair it. Meanwhile, methadone maintenance provides a safe and effective way to normalize the function of otherwise intractable opiate addicts.
In Dole’s view, the persistent receptor disorder is the result of chronic opiate use, leading to down-regulation of the modulating system and possibly also to suppression of the endogenous ligands. Goldstein (6) supported the concept of a metabolic disease as well as a genetic predisposition to that disease. Goldstein suggested that genetic influence carries an exceptional vulnerability to the disease in the presence of certain environmental influences. Kreek (19) suggested that multiple genes may account for different degrees of vulnerability to developing addiction. Other research also supports the view that heroin addiction has a genetic component (20–24). Further research is needed to define the metabolic disease process and the respective roles of genetic predisposition and environmental exposure.
Positron emission tomography scans that look at cerebral metabolism in opiate-dependent patients suggest that methadone maintenance at least partly normalizes cerebral glucose metabolism, as compared with patients withdrawn from methadone and in sustained remission (25). Magnetic resonance spectroscopy comparing methadone-maintained patients with different elapsed treatment times showed a nearly normal phosphorus metabolism profile in those patients who had been in treatment long term, suggesting healing at the neurochemical level over time (26). A key question for future research remains whether it is possible to restore normal functioning without maintenance therapy and, if so, how to accomplish this function.
CLINICAL ISSUES IN MAINTENANCE PHARMACOTHERAPY
Goals of Pharmacotherapy of Opioid Dependence
Kreek (19) outlined the goals of treatment and the properties of desirable opioid agonist medications as follows:
1. Prevention or reduction of withdrawal symptoms
2. Prevention or reduction of opioid craving
3. Prevention of relapse to use of addictive opioids
4. Restoration to or toward normalcy of any physiologic function disrupted by chronic opioid use
Profile of Potential Psychotherapeutic Agents
Characteristics of potential psychotherapeutic agents can be defined as follows:
1. Such medications are effective without requiring parenteral administration.
2. They have a long biologic half-life (>24 hours).
3. They have minimal side effects during chronic administration.
4. They are safe (i.e., they lack true toxic or serious adverse effects).
5. They are efficacious for a substantial proportion of persons with the disorder. Methadone and buprenorphine generally evince these characteristics.
MAINTENANCE TREATMENT USING METHADONE
Heroin versus Methadone
The opioid-dependent person who is actively misusing heroin or other short-acting opioids typically experiences rapid and wide swings from a brief pleasure usually characterized by sedation, fading into a period of normalcy and alertness, which can be described as the “comfort zone.” This period is followed by the beginnings of subjective withdrawal, sometimes called craving, which soon develops into the full objective withdrawal syndrome typical of opioid addiction. This cycle is particularly evident in the patient who engages in injection or inhalation of potent short-acting opioids such as heroin. A full cycle from “sick” (withdrawal) to “high” (intoxication) to “normal” (alert, comfortable) to “sick” (withdrawal) can occur repeatedly throughout the day (Fig. 50-1). The sensation of the “rush” is associated with a very rapid increase in blood levels, to a point somewhat above the therapeutic window. The pleasure is experienced during the time that drug levels remain above the therapeutic window (Fig. 50-2). Methadone, regularly administered at steady state, is present at levels sufficient to maintain alertness without craving or drug preoccupation (comfort zone or therapeutic window) throughout the dosing interval—usually 24 hours.
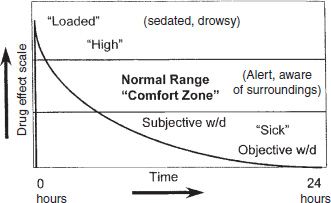
FIGURE 50-1 Heroin-simulated 24-hour dose–response.
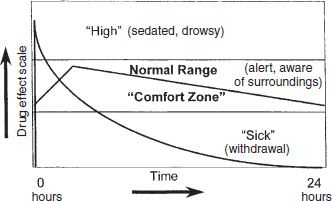
FIGURE 50-2 Methadone 24 hours at steady state.
With the next maintenance dose, there is a gradual rise in blood level, reaching a peak at 3 to 4 hours. Typically, the peak level is less than two times the trough level. There is a gradual decline over the rest of the 24-hour period, back to the trough level. When the patient is on the correct dose at steady state and with development of sufficient tolerance, at no time does the rate or extent of change in blood levels cause a sensation of being intoxicated or result in withdrawal symptoms.
Induction
Although most patients eventually will need 80 to 120 mg/d of methadone to achieve stability (11), and although adequately high doses are needed to provide stability and retention in treatment (27), the starting dose must be much lower, and the eventual steady state is reached slowly, sometimes over weeks. The first several doses require careful evaluation and adjustment. This phase is usually called induction and is the most critical phase of treatment. As treatment is made more available, an understanding of the pitfalls of this time of early treatment has become evident. Even though methadone maintenance has been shown to reduce mortality, including overdose mortality (28,29), several studies have reported deaths during the first 10 to 14 days of treatment, particularly when induction doses are high and when the patient is also ingesting sedatives (30–34). About 42% of drug-related deaths during treatment occurred during the first week of OMT (35). In an Australian survey, patients were reported to be 6.7 times more likely to die during induction as compared to untreated heroin users (36). The mean induction dose was more than 50 mg among those who had died. Such deaths also occur in the United States, though the maximum first dose is set at 30 mg by regulation. Variability in methadone metabolism, discussed later in the section on drug interactions, may be implicated.
Initial Dose
In most cases, patients being evaluated for admission to OMT have developed significant tolerance to opioids and demonstrate objective signs of withdrawal as a sign of current opioid physical dependence. The response to the initial dose of agonist medication provides valuable information about tolerance levels and the target “therapeutic window.” Significant relief during peak (2 to 4 hours) is evidence that the dose is in the range of the established level of tolerance and may not require further escalation. The absence of relief suggests that the dose is well short of the therapeutic window. Additional methadone can be provided when significant objective withdrawal persists during peak methadone levels. Patients who present at the dosing window 24 hours after their very first dose can be expected to be uncomfortable as tissue store accumulation is still incomplete. If they were comfortable during the first 4 to 12 hours after their dose, they probably need more time at the same dose, and not a higher daily dose. Under federal regulations, the initial dose of methadone is no more than 30 mg and may be lower in patients in whom low tolerance might be expected (e.g., recent relapse after a significant period of abstinence, or addiction to lower-potency opioids such as hydrocodone or codeine, or in opium smokers). A total dose of no more than 40 mg may be given on the first treatment day unless the program physician documents in the patient’s record that 40 mg did not suppress opioid abstinence symptoms.
Stabilization and Steady State
After the initial dose, the induction phase allows for subsequent careful adjustments of the dose to achieve elimination of drug craving and prevention of withdrawal while avoiding the risk of intoxication or overdose associated with accumulation of methadone (37,38). The induction phase can be considered to last until the patient has attained a methadone dose that meets the four goals outlined above. The safe and effective introduction of methadone requires an understanding of steady-state pharmacologic principles. In general, steady-state levels are reached after a drug is administered for four to five half-lives (methadone has an average half-life of 24 to 36 hours). The clinical significance is that, with daily dosing, a significant portion of the previous dose remains in tissue stores, resulting in increased peak-and-trough methadone levels after the second and subsequent doses. Thus, the levels of methadone increase daily, even without an increase in dose. The rate of increase levels off as steady state is achieved at four to five half-lives, that is, 3 to 7 days (Fig. 50-3). Further dose changes every 3 to 7 days may be needed to achieve the maintenance dose. Dose adjustments can be done in 5- to 10-mg increments for highly tolerant patients. Liquid medication allows dose adjustments by smaller increments for less tolerant patients.
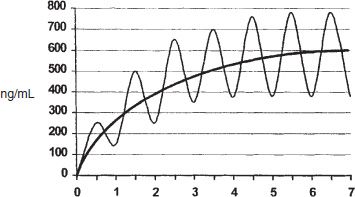
FIGURE 50-3 Steady-state simulation—maintenance pharmacology attained after 4 to 5 half-times, 1 dose/half-life.
Maintenance
Once a stable dose is established, based on the presence of desired clinical effects, elimination of craving, and prevention of withdrawal, the maintenance phase begins. Maintenance continues until such a time that there is a reason to alter the treatment. Most patients receiving methadone maintenance do well on a dose range of 80 to 120 mg/d (11), though some patients require less and some require more. A prospective study of methadone doses associated with heroin abstinence showed a wide range, with some of the variance attributable to mental health diagnosis, further supporting individualization of the dose (39). Patients are often able to remain at their maintenance dose for years with no need to adjust it. Tolerance appears to remain stable with no need to escalate the dose, as would be the case for short-acting opioid analgesics. Endocytosis of mu opioid receptors and N-methyl-D-aspartate receptor antagonism are unique characteristics of methadone itself that may contribute to this stabilizing feature (40–43).
Duration and Dose
Dose level and duration of treatment are individualized clinical decisions. For most patients in methadone maintenance, a chronic care approach is most appropriate. In the early 1970s, efforts to limit the duration of treatment and to cap the dose occurred initially at the federal level and later were initiated by some individual state methadone authorities (44). There is no scientific or clinical basis for an arbitrary dose ceiling on methadone, although QT prolongation has been seen in the electrocardiograms of patients receiving high doses of methadone (37). Methadone doses of 80 to 100 mg have greater benefits than doses below 50 in heroin-dependent patients (45–48). Based on an extensive review of the research literature on the prognosis of patients who have been withdrawn from methadone, as well as the safety of continued maintenance treatment, the American Society of Addiction Medicine supports the principle that methadone maintenance treatment is most effective as a long-term modality (49). Once the dose has been determined to be adequate, daily dose remains constant, and appropriate behavioral and psychosocial interventions can be effective.
The known risks of discontinuing methadone treatment, with predictable relapse to injected heroin use, become increasingly critical when viewed in the context of the HIV epidemic. These risks, when compared to the proven safety and efficacy of long-term methadone treatment, suggest that long-term—even indefinite—methadone treatment is appropriate and even essential for a significant proportion of eligible patients. Methadone treatment currently is viewed as treatment of a chronic medical disorder, with the goal of achieving control of the opioid addiction and avoiding the ravages of the untreated disease (50). Treatment should be continued as long as the patient continues to benefit from treatment, wishes to remain in treatment, remains at risk of relapse to opioid or other substance use, and suffers no significant adverse effects from continued methadone maintenance treatment and as long as continued treatment is indicated in the professional judgment of the physician (51). Patients receiving methadone do seek to discontinue maintenance for nonmedical but very real and practical reasons (e.g., transportation or scheduling difficulties) and to escape continued disruption of their lives associated with the burdensome restrictions, regulations, and structure of the treatment delivery system. For patients who attempt withdrawal, it is important for practitioners to provide encouragement along with the best medical and supportive treatment available, without fostering unrealistic expectations or unnecessary guilt, and to provide a means for rapid readmission to methadone treatment in the event of relapse or impending relapse to the use of illicit opiates (49).
Techniques to Ensure Adequacy of Dose
In most cases, clinical observation and patient reporting are adequate to make appropriate dose determinations.
Blood Levels in Dose Determination
Mean, random, or trough levels of methadone do not define an adequate dose. The clinical utility of blood levels is based on the peak and trough values to define a rate of change, or a peak-to-trough ratio. In the methadone clinic setting, patients occasionally experience problems in maintaining stability on a given dose of methadone. Statements such as “My dose isn’t holding me,” “I wake up sick every morning,” “My dose only lasts a few hours,” “I have drug hunger every night,” or “I get sleepy at work but start getting sick by bedtime” are not uncommon in patients receiving methadone. These clinical problems may not respond to simple dose adjustments and may suggest wide fluctuations over the dosing interval (rapid metabolism). As early as 1978, it was suggested that serial methadone levels could result in dramatic clinical improvement, with a “flattening of the curve” associated with a divided dose regimen in those methadone maintenance patients who were experiencing problems on a single daily dose (52). Researchers in the early 1980s compared 24-hour methadone serum levels in two groups of patients, all of whom received 80 mg/d. One of the groups was composed of patients who were doing very well in treatment, while the patients in the other group were doing poorly in terms of drug use and compliance. The results showed the stable group to have a mean serum level of 410 ng/mL at 24 hours, whereas the poor performers had a mean of 101 ng/mL (53). It has become clear that the same dose may vary in efficacy among individuals and that patients may be doing poorly as a result of inadequate dosing. Several researchers support blood levels greater than 150 to 200 ng/mL at all times for optimum results (7,54,55). There is growing consensus that levels above 400 ng/mL can represent an optimum level in providing adequate cross-tolerance to make ordinary doses of intravenous heroin ineffective (nonreinforcing) during methadone treatment (56). Methadone peak, trough, and mean levels and the rate of elimination (half-life) can be influenced by several factors. Individual differences in the metabolism of methadone, poor absorption, changes in urinary pH, effects of concomitant medications, diet, and even vitamins are among the possible factors that can influence the 24-hour dose–response curve of methadone. Pregnancy, particularly during the third trimester, is associated with significant decrease in trough methadone levels, suggesting increased rates of metabolism of methadone (57). Blood level assays can be very useful in evaluating suspected drug interactions. Blood levels can help identify patients who may benefit from a divided-dose regimen or demonstrate the effectiveness of a divided-dose regimen.
Procedure for Obtaining Blood Levels
Ideally, peak blood levels should be drawn at 3 (2 to 4) hours after a dose and trough levels at 24 hours, once said dose has been stable for at least 5 days, to allow tissue store equilibration. Patients already on a divided dose, such as every 12 hours, should have 2- to 3-hour and 12-hour specimens. A trough level alone is of little clinical value unless it is extremely low or very high. Blood levels are interpreted in the context of a clinical presentation for which the laboratory values can supplement clinical judgment. The peak level at 2 to 4 hours should be no more than twice the trough level. A peak-to-trough ratio of 2 or less is ideal (peak/trough = ratio). Ratios greater than 2 suggest rapid metabolism. The rate of change is of greater clinical significance than the actual levels. For example, a patient with a 24-hour level of 350 ng/mL after a peak of 1,225 ng/mL (1,225/350 = 3.5, indicating rapid metabolism) may be experiencing early opioid withdrawal, whereas a patient with a trough of 150 ng/mL and a peak of 250 ng/mL (250/150 = 1.7, indicating a normal metabolism) may be quite comfortable. No particular blood level should be considered “therapeutic” outside of the clinical context.
Methadone–Drug Interactions
Clinical experience suggests that concomitant medications can either induce or inhibit CYP450 activity on methadone metabolism (58). Drugs that stimulate or induce CYP450 activity can precipitate opioid withdrawal by accelerating metabolism, thus shortening duration and diminishing intensity of the effect of methadone. For example, addition of rifampicin, phenytoin, carbamazepine, phenobarbital, nevirapine, or efavirenz may result in the onset of withdrawal symptoms and require dose adjustments of the methadone. Considerable flexibility in dosing may be required to stabilize some patients whose metabolism has been altered by drug interactions or naturally occurring altered rates of metabolism. Other drugs such as cimetidine, ciprofloxacin, fluconazole, erythromycin, and fluvoxamine may inhibit this enzyme activity, slowing the metabolism and extending the duration of the drug effect. Metabolism of methadone is largely a function of enzyme activity in the liver, and intestinal enzymatic activity has also been observed to be clinically relevant. Multiple enzymes may affect methadone metabolism in vivo (59). Liver 3A4 activity in vitro is shown to influence methadone metabolism (60). Methadone exists as a racemic mixture of R and S isomers. Genetic variability in CYP 2D6 activity may affect clinical status by affecting metabolism of the R-methadone isomer (61,62) and may also affect toxicity (63). Enzymatic activity of intestinal CYP2B6 is stereoselective and may be important in clinical effects and drug interactions (64). A 17-fold variability between patients in their methadone metabolism is shown, mostly due to activity of various enzymes (65).
Methadone and QT Interval
Several case series have been published showing that methadone treatment is associated with prolongation of the QT interval on the electrocardiogram and possible consequent cardiac arrhythmia (Torsade des Pointes or TdP) (66,67).
In vitro study of human ether-à-go-go–related gene potassium channels (hERG K+) confirms that methadone at therapeutic doses can affect cardiac conduction (68). Genomics of CYP2B6 were shown to be associated with QT interval, and in vitro studies suggest that most of this prolongation is due to the nontherapeutic S-methadone enantiomer (69). Interviews of patients receiving methadone treatment found an association between longer QT and retrospective self-report of syncopal episodes (70).
In 2006, the U.S. Food and Drug Administration (FDA) published a boxed warning that included QT interval prolongation and the risk of arrhythmia. Although this warning was aimed at patients on high doses for pain treatment, clinicians in OTPs are becoming more aware of this risk.
Several studies look at QT interval in the methadone clinic setting. Martell et al. performed electrocardiograms on 160 patients admitted to methadone treatment at baseline and again after 6 months and 12 months of the treatment. The study found that admission to methadone treatment prolonged the QTc an average of 10 milliseconds at 12 months and that the prolongation correlated with serum levels of methadone (71). Peles et al. measured QT in 138 patients already on stable doses of methadone and found that three of them had QTc greater than 500 milliseconds, and during the study, two of those patients died, although the deaths were not judged to be cardiac. An additional 19 patients, all with doses greater than 120 mg, had QTc > 450 milliseconds (72). Both of these studies give an overall prevalence of prolonged QT interval (≥500 milliseconds) of around 2% in the OTP. A randomized controlled trial found that 23% of methadone patients had a QTc > 470 milliseconds (women) or 490 milliseconds (men) but did not find frequent QTc prolongation among patients treated with buprenorphine (73). Physicians remain underaware of cardiac safety concerns about methadone (74), but many are adapting to this information in various ways. There are no clear data to guide any intervention. Some clinics are offering cardiograms and are screening patients for cardiac risk. Risk–benefit discussion with patients includes a review of other medications that might contribute additional cardiac risk. In general, it is considered that almost certain relapse to uncontrolled opioid use is more risky than the rare occurrence of an arrhythmia. However, coordination of care with outside physicians to monitor the use of other medications or transfer to buprenorphine treatment might in some cases be indicated (75). Hospitalized patients receiving methadone may have particularly high risk of TdP (75). The Center for Substance Abuse Treatment (CSAT) convened a consensus panel on this topic, and the final iteration of the consensus panel’s recommendations include risk assessment and ECG screening, patient and staff education about cardiac risk, and informed consent (76,77). Feasibility of offering QT screening on-site in the OTP has been shown in several studies (78,79).
Methadone-related Deaths of Persons Not in Treatment
The increase in methadone abuse parallels the increase in prescription analgesic abuse noted since 1995, with associated mortality from nonmedical ingestion of methadone, in particular a dramatic increase in mortality by ingestion of diverted methadone intended for pain treatment. Deaths from methadone overdoses exceeded deaths from heroin in some states by 2002 (80). In 2003, the CSAT published an analysis of an increase in deaths related to methadone in the United States, and in 2007 and 2010, a second and third review (81,82). Most of these deaths involved diverted methadone intended for pain treatment and were not patients in maintenance treatment, nor did it appear that the methadone was diverted from that dispensed at the OTPs. Most of these deaths also involve multiple medications, usually including sedative medication. These observations are similar to observations of increased diversion-related deaths after ingesting methadone in other countries where OMT was initiated (83–85).
Levo-Alpha Acetylmethadol
Levo-alpha acetylmethadol (LAAM) was developed in 1948. By 1952, it had been observed to suppress opioid withdrawal for more than 72 hours (86). LAAM was evaluated in opioid addiction in the late 1960s and 1970s, ignored in the 1980s, and resurrected in 1990 by the Medications Development Division of the National Institute on Drug Abuse (87). LAAM was approved for use in the treatment of opioid addiction by the FDA in 1993 (88). After several cases of the arrhythmia Torsade des Pointes were reported, the FDA placed a boxed QT warning label on LAAM, and subsequently the pharmaceutical company withdrew LAAM from the market. As of this writing, it remains unavailable in the United States, though still approved as an opioid agonist medication for OMT.
MAINTENANCE TREATMENT USING BUPRENORPHINE
Buprenorphine is a partial mu opioid agonist. In October 2002, the FDA approved two sublingual formulations of buprenorphine for treatment of opioid dependence. One formulation, a combination of buprenorphine and nalox-one in a 4:1 ratio, is designed to discourage injected diversion and misuse. Naloxone is an opioid antagonist that is not significantly bioavailable when taken sublingually or when swallowed. When injected into actively using opioid-dependent subjects who are blinded, the combination was not judged to be desirable or to be different from the antagonist in the first hour after injection (89,90). This buprenorphine/naloxone combination is the preferred formulation for outpatient use as an effort to minimize diversion. The other sublingual formulation contains only buprenorphine and is used in controlled settings, such as inpatient medically supervised withdrawal, or in pregnancy. Sublingual buprenorphine/naloxone maintenance treatment is usually offered in office-based settings. Sublingual buprenorphine is listed as an opioid treatment medication under federal regulations that govern OTP licensure, although take-home criteria are less restrictive than for methadone.
Pharmacology of Sublingual Buprenorphine
Buprenorphine has slow onset and long duration of action, conferring similar maintenance benefits as discussed earlier for methadone. As a partial mu agonist, buprenorphine has a maximal dose–effect ceiling that is well below significant respiratory depression for most patients. This safety profile led to its DEA Schedule III, allowing office-based use under the restrictions of the Drug Addiction Treatment Act of 2000 (91).
Induction and Precipitated Withdrawal
Buprenorphine is a partial agonist with strong receptor affinity. Relative to a displaced full agonist, its activity can be felt as the rapid onset of (relative) opioid withdrawal by the patient unless the first dose is clinically well timed. The first dose should be given when the patient is already in obvious opioid withdrawal. When opioid withdrawal is already present, the onset of activity will be felt as agonist, with relief of withdrawal. In contrast to methadone, induction doses are not set by regulation, though clinical guidelines and physician training courses recommend 2 to 4 mg of sublingual buprenorphine/naloxone as a first dose, with first-day maximum of 8 mg (92). One Italian study found that higher induction doses were associated with better engagement in ongoing care (93).
Buprenorphine Dose Adjustment
The dose can be rapidly titrated over the first 3 days to control withdrawal. Average daily doses are 16 to 20 mg. One labeled imaging study showed mu opioid receptors to be approximately 90% occupied depending upon the brain region at doses of 16 mg/d of sublingual solution (94). Because of the partial agonist ceiling effect, no additional maintenance benefit is expected in doses above 32 mg/d. Sublingual buprenorphine/naloxone is usually prescribed as a single daily dose. In cases requiring supervised dosing, it can be given every other day, or three times a week, while keeping the total weekly dose unchanged (95,96).
Buprenorphine Medication Interactions
Compared to methadone, buprenorphine may confer advantages when certain HIV medications are used (97) and in cases of QT prolongation with methadone (73,75). Even though it is metabolized by the liver in a manner similar to that of methadone, by CY450 3A4, the presence of an active metabolite—norbuprenorphine—and the strong receptor attachment make this medication less dependent than methadone on blood level and tissue stores.
Federal Regulations and Sublingual Buprenorphine
When dispensed in the OTP, sublingual buprenorphine/ naloxone is subject to the same regulations as methadone, except for unobserved dosing requirements, as explained below. When prescribed in the office-based setting, there are certain restrictions set forth in the Drug Addiction Act of 2000 (91). This law provides for a waiver to the 1914 Harrison Act that forbids prescription of a narcotic to an addicted person. The qualifying physician notifies the Secretary of Health and Human Services of his or her intent to prescribe, after which the DEA assigns to the physician a second DEA number that is specifically for use under DATA 2000. There are restrictions on census and type of medication and rules on storage and record keeping. The DATA 2000 restrictions do not apply when buprenorphine or buprenorphine/naloxone are dispensed at clinics under their OTP license. In those cases, the same federal regulatory restrictions apply to both buprenorphine and methadone except that the time-in-treatment regulations that apply to take-home doses of methadone no longer apply for buprenorphine so that decisions about the number of buprenorphine take-home doses given are determined purely on the basis of patient stability and not on how long the patient has been in treatment.
Diversion and Abuse of Buprenorphine
An investigation of the effects of DATA 2000 was carried out in 2005. No serious adverse events were found by the introduction of sublingual buprenorphine/naloxone in the office-based setting. That study showed that most of the patients treated with buprenorphine listed prescription pain relievers as their primary opioid of abuse (98). In view of the serious increase in nonmedical usage of prescription opioids, it is hoped that buprenorphine/naloxone may provide a timely treatment for those who become addicted and who might not otherwise seek out a methadone clinic.
Wherever buprenorphine treatment has been introduced, there has been diversion and abuse of the medication, including injected use (99,100). As the clinical use of buprenorphine has increased since 2002, so have reports of diversion. One study in the United States showed that diverted buprenorphine/naloxone is being used mostly for relief of withdrawal and rarely as a primary drug of abuse (101). A more recent study found that diversion increased as treatment with buprenorphine became more available, although not as steeply as full agonists (102). In areas of higher prescribing of sublingual buprenorphine, most treatment seekers have already tried buprenorphine/naloxone, having used it illicitly for relief of withdrawal, or during times when the preferred drug of abuse is not available (103). An Australian study that interviewed patients who were found to be diverting doses given under observation at pharmacies found that discarding it, saving it for another time, or giving the dose to someone else were cited as reasons for not correctly taking the dose at the window (104,105). Treatment guidelines for agonist treatment recommend monitoring for diversion with urine testing or pill counts and adding observed dosing or shortening time span between prescriptions when diversion is suspected.
Benzodiazepines and OMT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


