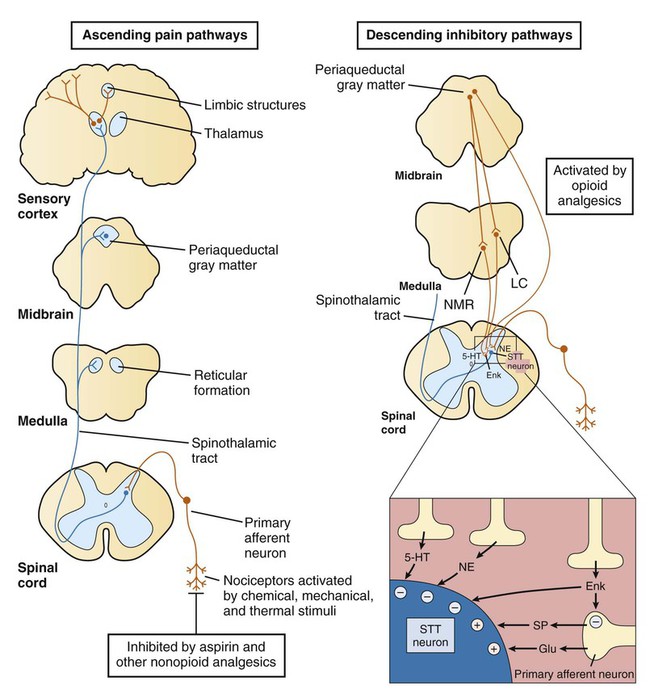
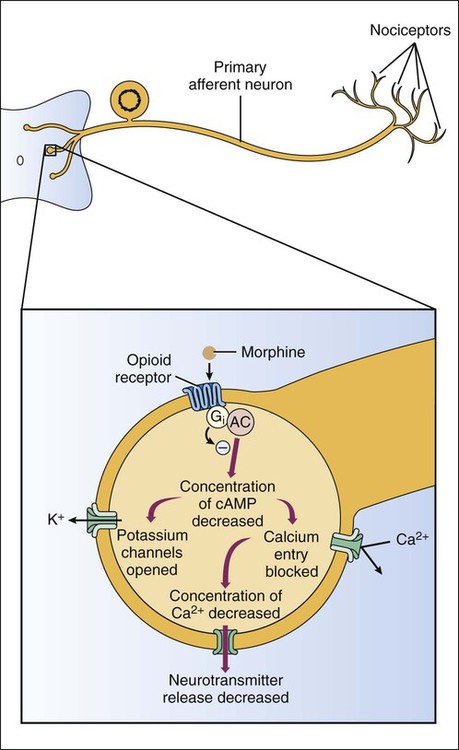
Based on their mechanisms of action, analgesics can be classified as opioid analgesics or nonopioid analgesics. Opioid analgesics act primarily in the spinal cord and brain to inhibit the neurotransmission of pain. In contrast, nonopioid analgesics act primarily in peripheral tissues to inhibit the formation of algogenic or pain-producing substances such as prostaglandins. Because most of the nonopioid analgesics also exhibit significant antiinflammatory activity, they are called nonsteroidal antiinflammatory drugs (NSAIDs). The NSAIDs are described in greater detail in Chapter 30. To facilitate the selection of an appropriate analgesic or anesthetic medication, patients are usually asked to describe their pain in terms of its intensity, duration, and location. In some cases patients report an intense, sharp, or stinging pain. In other cases they describe a dull, burning, or aching pain. These two types of pain are transmitted by different types of neurons and their primary afferent fibers (Box 23-1). Pain can be further distinguished on the basis of whether it is somatic, visceral, or neuropathic in origin. Somatic pain is often well localized to specific dermal, subcutaneous, or musculoskeletal tissue. Visceral pain originating in thoracic or abdominal structures is often poorly localized and may be referred to somatic structures. For example, cardiac pain is often referred to the chin, neck, shoulder, or arm. Neuropathic pain is usually caused by nerve damage, such as that resulting from nerve compression or inflammation, or from diabetes. Neuropathic pain is characteristic, for example, of trigeminal neuralgia (tic douloureux), postherpetic neuralgia, and fibromyalgia. As shown in Box 23-1, the primary afferent fibers transmitting nociceptive information are Aδ fibers and C fibers, which are responsible for sharp pain and dull pain, respectively. Spinal reflexes activated by these fibers can lead to withdrawal from a noxious stimulus before pain is perceived by higher structures. Ascending pain pathways consist of two main anatomic-functional projections: the sensory-discriminative component, to the cerebral cortex, and the motivational-affective component, to the limbic cortex. Projections to the sensory cortex alert an individual to the presence and anatomic location of pain, whereas projections to limbic structures (e.g., the amygdala) enable the individual to experience discomfort, suffering, and other emotional reactions to pain. The descending inhibitory pathways arise from periaqueductal gray (PAG) in the midbrain, and they project to medullary nuclei that transmit impulses to the spinal cord (see Box 23-1). The medullary neurons include serotonergic nerves arising in the nucleus magnus raphe (NMR) and noradrenergic nerves arising in the locus ceruleus (LC). When these nerves release serotonin and norepinephrine in the spinal cord, they inhibit dorsal spinal neurons that transmit pain impulses to supraspinal sites. Nerve fibers from the PAG also activate spinal interneurons that release an endogenous opioid peptide, met-enkephalin. The enkephalins act presynaptically to decrease the release of pain transmitters from the central terminations of primary afferent neurons. They also act on postsynaptic receptors on spinothalamic tract neurons in the spinal cord to decrease the rostral transmission of the pain signal. Opioid analgesics activate the descending PAG, NMR, and LC neuronal pathways, and they also directly activate opioid receptors in the spinal cord. The opioid drugs can be classified as full agonists, mixed agonist-antagonists, or pure antagonists. The opioid receptors are prominent members of the G protein–coupled receptor superfamily. Activation of opioid receptors leads to inhibition of adenylyl cyclase and a decrease in the concentration of cyclic adenosine monophosphate, an increase in K+ conductance, and a decrease in Ca2+ conductance (Fig. 23-1). The activated Gai subunit of the G protein directly inhibits the adenylyl cyclase enzyme, and the Gβγ subunits are thought to mediate the changes at the Ca2+ and K+ channels. These actions cause both presynaptic inhibition of neurotransmitter release from the central terminations of small-diameter primary afferent fibers and postsynaptic inhibition of membrane depolarization of dorsal horn nociceptive neurons. Morphine acts in the CNS to produce analgesia, sedation, euphoria or dysphoria, miosis, nausea, vomiting, respiratory depression, and inhibition of the cough reflex (Box 23-3). Analgesia is produced by activation of opioid receptors in the spinal cord and at several supraspinal levels, as illustrated in Box 23-1. Sedation and euphoria can be caused by effects on midbrain dopaminergic, serotonergic, and noradrenergic nuclei. Surprisingly, many patients experience dysphoria after administration of opioids. Miosis (constricted pupils) is produced by the direct stimulation of the Edinger-Westphal nucleus of the oculomotor nerve (cranial nerve III), which activates parasympathetic stimulation of the iris sphincter muscle. Because little or no tolerance develops to miosis, this sign can be diagnostic of an opioid overdose. Codeine and other opioids inhibit the cough reflex at sites in the medulla where this reflex is integrated. The antitussive actions of opioids are discussed in greater detail in Chapter 27. Morphine and most other opioids act to increase smooth muscle tone in the gastrointestinal, biliary, and genitourinary systems. In the gastrointestinal tract, increased muscle tone leads to inhibition of peristalsis and causes constipation. For this reason the opioids are the oldest and most widely used medication for the treatment of diarrhea (see Chapter 28). Unfortunately, patients with chronic pain do not appear to become tolerant to the constipating effects of opioids, necessitating a continual need for laxatives and other agents.
Opioid Analgesics and Antagonists
Pain and Analgesic Agents
Pain Pathways
Opioid Drugs
Classification
Drug Properties
Mechanism of Action
Pharmacologic Effects
Central Nervous System
Gastrointestinal, Biliary, and Genitourinary System
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Opioid Analgesics and Antagonists
Only gold members can continue reading. Log In or Register to continue