Fig. 13.1
Molecular structures of fluorescein sodium and wavelength spectra peak excitation and emission
The dye is visualized when illuminated in blue filter, appears florescent green, and is detectable in concentration between 0.1 and 0.0000001 % topically. For angiographic studies, sodium fluorescein is used extensively in clinical settings. The dose of 10–25 % fluorescein is injected through peripheral vein in a single bolus dose. Dye diffuses out rapidly from the capillaries except the retinal and central nervous system vasculature due to tight capillary junction in these location. On intravenous injection, 70–80 % of dye molecules bind to plasma proteins, and the remaining free or unbound molecules fluoresce on excitation by appropriate exciting wavelength (Morris 2002). It undergoes both hepatic and renal metabolism but is excreted principally through urine within 24–36 h of the administration. As it is small molecule, it can be excreted through milk also; therefore, its administration should be avoided in lactating mothers.
13.1.1 Ophthalmic Use
The primary purpose of fluorescein staining is to detect epithelial defects and to assist in the diagnosis of erosions, corneal abrasion, and keratitis. It has been a useful dye in assessing the rate of healing of corneal epithelial lesions. Fluorescein staining is also used to evaluate the status of the precorneal tear film with respect to tear breakup time (BUT) (Norm 1969) and contact lens fitting (Wolfe 1986) to evaluate tear volume and clearance, to measure corneal epithelial and endothelial permeability (Lass et al. 1985 and Kim 2000), and to detect aqueous humor flow and leakage, (Seidel test).

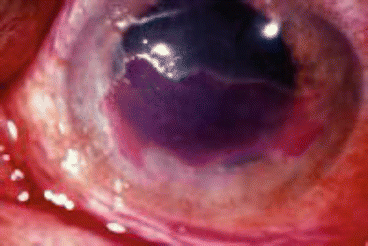
Image 13.1
Infectious epithelial keratitis stained with fluorescein
Fluorescein dye is also used to evaluate the tear secretion and tear turnover on the ocular surface in the fluorescein clearance test (Mishima et al. 1966). After a standardized amount of fluorescein is placed in the conjunctival sac, its concentration in the tear fluid is collected on Schirmer test strips placed in the inferior conjuctival fornix at 10-min intervals and visually compared to photographic standards. A reduced tear turnover rate is diagnosed by persistent fluorescein staining of the Schirmer test strips (Benedetto et al. 1984). Staining is enhanced with cell degeneration or death, which increases membrane permeability and manifests whenever there is a disruption of cell–cell junctions. Its staining is not blocked by tear components such as albumin and mucin or tear substitutes such as carboxycellulose (Feenstra and Tseng 1992). In addition, fluorescein sodium is routinely used for applanation tonometry, to check the patency of lacrimal passageways. Sodium fluorescein is extensively used in the retinal angiography to rule out ischemic, neovascularized, or obstructive pathology of the retina and choroid of the eye. (Table 13.1).
Table 13.1
Clinical uses of sodium fluorescein in ocular conditions
1. | Lacrimal system | (a) Tear breakup time (dry eyes) (b) Jones test, dye disappearance test (blockage of lacrimal system) |
2. | Cornea | (a) Detect epithelial defect (corneal abrasion, superficial punctate keratopathy) (b) Seidel’s test (wound leak) (c) Contact lens fitting |
3. | Anterior chamber | (a) Applanation Tonometry (b) Detect iris neovascularisation (c) TBUT (d) Clearance test |
4. | Retina | (a) Fluorescein angiography |
13.1.2 Adverse Effects
Sodium fluorescein is well tolerated by most patients, but angiography is an invasive procedure with an associated risk of complication or adverse reaction. Transient nausea and occasional vomiting are the most common reactions and require no treatment (Montavale 2006). These mild reactions typically occur 30–60 s after injection and last for about 1–2 min. More basic pH of this dye leads to painful experience at the site of injection, simultaneously extravasations of fluorescein dye can cause severe complications in the some patients although it is rare.
13.2 Trypan Blue
Trypan blue (TB) is a vital dye, first synthesized in 1904 by German scientist Paul Ehrlich. Trypan blue is an azo-based, hydrophilic, tetrasulfonated blue acid dye. Trypan blue is derived from toluidine which is a derivitized product of toluene, which has molecular formula of C34H28N6O14S4 with the molecular weight of 872.88 g mol−1. Trypan blue derived its name from trypanosomes, a parasite which causes sleeping sickness. An analog of trypan blue, suramin, is used for the treatment of trypanosomiasis (Morgan et al. 2011). This dye is also known as diamine blue and Niagara blue (Fig. 13.2).
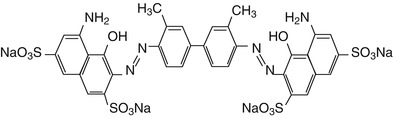

Fig. 13.2
Molecular structure of trypan blue dye
Trypan blue is used as cell membrane-staining dye and extensively used to determine the viability of cells. Trypan blue will stain dead cells with permeable membrane blue, while the dye is excluded by most living cells and their intact membranes thereby allowing visual determination of living versus dead cells.
13.2.1 Ophthalmic Utilization
TB is used in both penetrating and deep lamellar keratoplasties. For penetrating keratoplasty, 0.02 % TB solution may be injected into the anterior chamber via a paracentesis to stain the descemmets membrane (DM) of the donor as well as the recipient corneal tissue. Dye exposure may promote close alignment of edges of host and donor DM, thus improving graft stability and minimizing surgically induced astigmatism (Roos and Kerr Muir 2005).
The vital dyes Indocynine Green (ICG) and TB aid in visualization of conjunctival cyst capsules (Kobayashi et al. 2002). Intraoperative injection of TB provides various advantages in cataract surgery. Surgical TB injection may promote higher rates of success of capsulorhexis in phacoemulsification for cases with inadequate red reflexes (Kothari et al. 2001 and Nodarjan et al. 2001). The blue vital stain allows staining of anterior and posterior capsules in children younger than 5, thereby enhancing effectiveness for completing the Continous Curvilinear Capsulorhexis (CCC) (Brown et al. 2004 and Saini et al. 2003). Wollensak and coauthors found TB staining led to an increase in elastic stiffness and a significant decrease in ultimate extensibility in the anterior capsule. This effect was probably due to the photosensitizing action of TB to physical cross-linking of collagen through yielding of free oxygen radicals, which causes a change in elastic behavior (Satofuka et al. 2004). Most clinical studies prefer 0.1 % TB to stain the anterior capsule, but effective staining has been also achieved with concentrations as low as 0.06 % (Chang et al. 2005). The minimum incubation time reported for successful capsule staining ranges from 5 seconds to 2 min (Saini et al. 2003). Trypan blue is also predominately used for assessing cell viability of cultured cells (Stevens 1993).
13.2.2 Adverse Effects
Side effects reported include discoloration of high water content hydrogen intraocular lenses; a relative contraindication for TB is the use of hydrophilic expandable acrylic intraocular lenses (IOLs), which have the highest water content (73.5 %) of currently manufactured implants and inadvertent staining of the posterior lens capsule and vitreous face followed use of trypan blue. Staining of the posterior lens capsule or staining of the vitreous face is generally self-limited, lasting up to one week.
13.3 Rose Bengal
Rose bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein) was originally prepared in 1884 by Gnehm. It is an iodine derivative of fluorescein which has vital staining properties; it is also called true histological stain. This is also a hydroxyanthene dye of a similar molecular structure to fluorescein. Additional halides give it its red color and also render it toxic. This toxicity is further enhanced by exposure to visible light. Rose bengal is classically well known to stain dead and devitalized cells, but now it is also reported to stain nuclear components of healthy cells (Fig. 13.3).
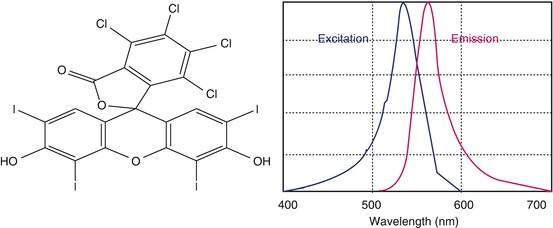

Fig. 13.3
Chemical structure of rose bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein) and fluorescence spectra (depicted)
13.3.1 Ophthalmic Uses
It stains dead or degenerated epithelial cells, not normal cells, and is used to help in the diagnosis of corneal abrasion, keratitis, keratoconjunctivitis sicca, lagophthalmos, and meibomian gland dysfunction. In meibomian gland dysfunction, even though the tear volume appears to be normal, rose bengal staining may occur on the inferior or superior bulbar conjunctiva under the eyelids, which is outside the exposure zones (Uchiyama et al. 2007). With more severe inflammation, staining spreads and may affect the cornea in addition to the exposed conjunctiva. In nocturnal lagophthalmos, rose bengal staining is limited to the inferonasal corneal and conjunctival epithelium with a discrete line of demarcation between stained and unstained tissue. In contrast, rose bengal stains only the affected superior bulbar conjunctiva and the superior cornea in superior limbic keratoconjunctivitis.
13.3.2 Adverse Effects/Toxicity
Rose bengal has also been known to have intrinsic cellular toxicity. Studies have shown that rose bengal has a dose-dependent, toxic effect on human corneal epithelial cells in vitro that is further enhanced by light exposure. Lastly, it’s widely known to cause patient discomfort, particularly stinging sensation upon instillation, which can become severe, is often a deterrent from using rose bengal.
13.4 Indocyanine Green
Initially developed by Kodak Research Laboratories in 1955 for the near-infrared (NIR) photography, indocyanine green dye was approved for clinical use in 1956 (Engel et al. 2008; Bjoronsson et al. 1982). The chemical name for indocyanine green is 1 H benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-,hydroxide, inner salt, sodium salt. For retinal angiography, it has been used from early 1970s. ICG absorbs mainly between 600 nm and 900 nm and emits fluorescence between 750 and 950 nm. Near-infrared light is known to penetrate biological tissues such as skin and blood more efficiently than visible light therefore, they are best suited for tissue imaging (Fig. 13.4).
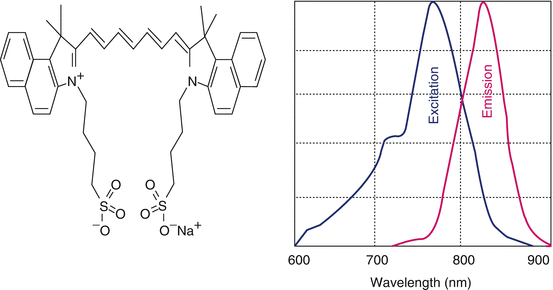

Fig. 13.4
Molecular structures of indocyanine green and its fluorescence spectra (depicted)
13.4.1 Ophthalmic Uses
ICG adheres well to the extracellular matrix components of the ILM, such as collagen type 4, laminin, and fibronectin (Wilson 1976). The first use of ICG in Macular Hole (MH) surgery was with a dye concentration of 0.5 %. After RPE changes and visual field defects appeared, a lower concentration was thought to be safer (T John 2003). ICG has been also used to facilitate Inner Limiting Membrane (ILM) peeling in other diseases: ICG-assisted ILM peeling in diabetic macular edema, proliferative diabetic vitreoretinopathy (PDVR), idiopathic Epiretinal Membrane (ERM) and proliferative vitreoretinopathy (PVR).
ICG is principally used for choroidal angiography. ICG is injected intravenously and circulate to reach the choroidal and retinal circulation. Due to its nature, ICG stays in the retinal and choroidal vessels; this allows the distinct outlines of the vessels of the choroid to be seen and identified. ICG is sometimes used to complement fluorescein angiography (FA). FA is often referred to retinal angiography, while ICG angiography is referred to choroidal angiography.
13.4.2 Adverse Effects/Toxicity
ICG is mainly metabolized by the liver and excreted through bile ducts; its toxicity is low as it is not absorbed through intestinal mucosa. Under UV exposure, ICG dissociates into a number of toxic substances which are still unknown substances (Toczylowska et al. 2014). The intravenous LD50 values measured in animals were 60 mg/kg in mice and 87 mg/kg in rats (Laperche et al. 1977). Its usage has been reported with analphylactic reactions, hypotension, tachycardia, dyspnea, and urticaria only in individual cases.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree