Fig. 6.1
Autonomic and trigeminal ocular projections in the eye (Adapted with permission from McDougal and Gamlin (2015))
Table 6.1
Presence and function of autonomic receptors in the structures of the eye
Structure | Sympathetic receptors | Response | Para-sympathetic receptors | Response |
|---|---|---|---|---|
Iris-radial muscle | α1 | Mydriasis | – | – |
Iris-spincter muscle | – | – | M3 & M2 | Miosis |
Ciliary muscle | β | Relaxation (far vision) | M3 & M2 | Contraction (near vision) |
Ciliary epithelium | α2 | Decrease production of aqueous humor | M1 & M2 | Unknown |
B2 | Production of aqueous humor | |||
Lacrimal glands | α1 | Secretion (+) | M3 & M2 | Secretion (3+) |
Corneal epithelium | B2 | Epithelial wound healing | M2, M4 & M5 | Unknown |
α1 | Cl-transport | |||
Corneal endothelium | β2 | Corneal hydration | M2, M4 & M5 | Unknown |
Trabecular meshwork | β2 | Decreased resistance | M3 | Increased resistance |
Retina and choroid (blood vessels) | β | Vascular control | M1–M5 | |
Conjunctiva | M3 | Goblet cell secretion | ||
Retinal pigmented epithelium | A1 & β2 | H2O transport | – |
6.2 Autonomic Control on the Pupil
Iris circular and radial muscles are innervated by postganglionic parasympathetic and sympathetic nerves respectively. Upon the activation of sympathetic nerves, norepinephrine is released from the postganglionic nerve ending, causing radial muscles of the iris to get contracted, causing mydriasis, whereas parasympathetic activation releases acetylcholine (ACh) from postganglionic nerve terminals causing miosis. The mechanism of them causing mydriasis and miosis are shown in Fig. 6.2. The dynamic equilibrium between both of the nervous systems determines pupillary diameter based upon the amount of light reaching the retina. The normal human eye can dilate the pupil from 1.5 to 8 mm in diameter and this change is expected to modulate the depth of focus, optical aberration, retinal illumination, and diffraction. Coordinated performance of the iris aperture along with focal length adjustment rendered by ciliary muscles by autonomic control is required for the optimum performance of visual system at different conditions. When the dynamic equilibrium exists between the sympathetic and parasympathetic system, pharmacological agents are used to achieve mydriasis or miosis by stimulating the appropriate autonomic receptors. Phenylephrine is an alpha-1 agonist capable of stimulating the contraction of iris radial muscle and cause mydriasis but atropine which is a muscarinic blocker is capable of removing the influence of cholinergic stimuli-induced contraction on iris circular muscle (sphincter muscle), thereby causing the domination of existing tone of sympathetic system leading to mydriasis. Dapiprazole is a selective α(1)-adrenoreceptor antagonist approved to reverse the mydriasis induced by atropine.
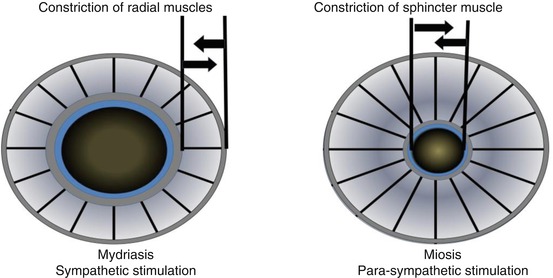

Fig. 6.2
Showing the effect of adrenergic (sympathetic) and cholinergic (parasympathetic) stimulation on pupillary diameter causing mydriasis and miosis respectively
Similarly, miosis can be induced by the stimulation circular muscles of the iris (sphincter muscle) using cholinomimetic-like pilocarpine or cholinesterase inhibitors and α(1)-adrenoreceptor blocker dapiprazole. Dapiprazole causes miosis by removing the action of sympathetic tone on radial muscles of the iris, leading to the shift of equilibrium to cholinergic tone (Bartlett and Classe 1992).
Apart from the presence of acetylcholine (neurotransmitter of cholinergic nerves) and norepinephrine (neurotransmitter of sympathetic nerves), other neurotransmitters and neuropeptides such as VIP, neuropeptide Y, nitric oxide synthase (for nitric oxide), and ATP were identified in the postganglionic neurons; they are not exploited so far by any pharmacological means for diagnostic or therapeutic potential. However, they are reported to be involved in the intrinsic pathways in controlling blood supply, aqueous humor dynamics, and other functions (Neuhuber and Schrodl 2011). The list of adrenergic and cholinergic receptors identified in various ocular structures and their functional importance are shown in Table 6.1. Apart from the classical autonomic pathway, morphine is known to stimulate parasympathetic pathway through the Edinger-Westphal nucleus, causing miosis in the iris.
Anisocoria is a condition commonly manifested by the unequal sizes of pupils. This could vary from simple to life-threatening conditions. Pupillary inequality is usually identified by their reaction in both dim and bright illuminations before initiating further clinical proceedings. Once the dysfunctioning of pupils is confirmed, differential diagnostic procedure is opted in neuro-ophthalmology in defining the underlying condition. It is aided by a handful of drugs like pilocarpine, hydroxyl-amphetamine, cocaine, phenylephrine, and morphine.
6.3 Autonomic Control Over Accommodation
Accommodation in the eye is primarily due to the strong influence of the parasympathetic system. The lens in the anterior segment is enclosed in a lens capsule and suspended by the specialized fibers called zonules from the ciliary body. Ciliary muscles are clarified into outer longitudinal, middle radial, and inner circular layers. In normal conditions influence of the parasympathetic system makes the ciliary circular muscles to contract, causing zonules to relax and thereby causing the lens to become convex and move a little toward the anterior segment. This action fixes the lens toward for near vision, called accommodation. When the radial muscle is pulled by sympathetic stimulation, stretched zonules cause reduction in curvature, leading to the lens adjustment for far vision. Antimuscarinic drugs like atropine abolish the effect of accommodation, causing the lens to fix for far vision. One of the mechanisms put forward for sympathomimetic agents is that their action on ciliary radial muscle puts traction on scleral spur, causing a decreased resistance in trabecular meshwork in reducing intraocular pressure.
6.4 Autonomic Control Over Lacrimal Secretion
Contribution of lacrimal gland secretion plays an important role in the formation of precorneal tear fluid. Parasympathetic innervation through M3 receptors plays a major role in the lacrimation and it is modulated by the sympathetic system. The lacrimal gland is reported to have transcellular Cl− secretion in the acinus which involves Cl−-selective channels in the apical plasma membrane (Mircheff 1989). Muscarnic agonist carbachol increases transepithelial Cl− fluxes that attribute to lacrimal fluid production (Selvam et al. 2013). Interestingly, some degree of cross-talk between purinergicP2 receptors and M3 and α1D-adrenergic receptors are reported to influence tear secretion (Murakami et al. 2000). Purinergic receptor subtypes are known to play divergent physiological functions in various structures like in corneal wound repair, modulating trabecular remodeling, visual processing in the retina, etc. (Sanderson et al. 2014). Pilocarpine is known to stimulate lacrimation through muscarinic receptors of the lacrimal gland and atropine is known to block lacrimal secretion and causes dry eye. Antihistaminics are known to have antimuscarinic side effects which are mediated through M3 receptor responsible for glandular secretion. Therefore, the lacrimal, P2Y(2), receptor subtype gains pharmacological importance due to its involvement in sharing the tear secretion pathway like carbachol. Topical instillation of P2Y(2) receptor agonist (diquafosol) has been reported to increase in net Cl−, fluid transport, and glycoprotein release onto the ocular surface (Fujihara et al. 2001; Mundasad et al. 2001).
6.5 Autonomic Control Over Aqueous Humor Production and IOP Control
In the anterior segment of the eye, aqueous humor produced by the ciliary processes is secreted into the posterior chamber and it reaches into anterior chamber through the pupil. From the anterior chamber, majority of the aqueous humor (80–95 %) drains into the canal of Schlemm to the episcleral venular plexus and finally it drains into systemic circulation. Apart from this route, it also routes through unconventional uveoscleral pathway. The rate of production of aqueous humor and its disappearance through drainage pathways together determines intraocular pressure and finally development of glaucoma. Production of aqueous humor comes under the influence of the sympathetic system through beta-receptors which is regulated by presynaptic α2 receptors (autoreceptors) in the sympathetic nerve endings. The classical regulation of intraocular pressure is achieved by blocking β2 adrenergic receptors or by stimulating presynaptic α2 receptors. Apart from this mechanism, directly or indirectly acting sympathomimetics widen the trabecular meshwork by creating a traction in the scleral spur through ciliary muscles. Epinephrine has also been reported to increase uveoscleral flow, which is likely through stimulating beta-2-adrenergic receptors (Almand Nilsson 2009).
The sympathetic system supplies to tissues of eyes through its various receptors and thus may produce diverse effects depending on the receptor activated. It supplies the pupil dilator muscle and brings about mydriasis through α1 receptor. It also produces the contraction of Muller’s muscle through the same receptors. The sympathetic system has dual effect on aqueous production wherein it enhances the aqueous humor formation through ß2 receptors while inhibiting formation through α2 receptors. It also relaxes the ciliary body muscle through its ß2 receptors. The parasympathetic ocular effects are produced through muscarinic receptors. This includes supply to the sphincter papillae muscle, causing miosis and contraction of the ciliary body muscle, leading to accommodation and enhanced drainage of aqueous humor.
6.6 Autonomic Control Over Blood Circulation and Neuroprotection
Alpha-2 adrenergic agonists, in addition to their pressure-lowering effects in the eye, may act directly upon retinal neurons, including retinal ganglion cells. Alpha-2A expression was identified in the human retina, on ganglion cells, and cells in the inner and outer nuclear layers (Kalapesi et al. 2005), whereas only a few clinical studies so far showed any benefit of neuroprotection in glaucoma (Evans et al. 2003; Sena and Lindsley 2013). Presence and involvement of β-adrenergic system in angiogenic processes has been implicated in infantile hemangiomas, retinopathy of prematurity, and cancer (Filippi et al. 2015). As the neurotransmitters of the autonomic nervous system such as acetylcholine and norepinephrine are vasoactive substances, therefore, presence of adrenergic and cholinergic receptors at the cornea, iris, ciliary body, retina, and choroid is expected to play a significant role on their vascular tone and cellular homeostasis (Mori et al. 2010; Toda et al. 1996; Casini et al. 2014). However, the current knowledge available is not enough to speculate the pharmacological utilization of these receptors in various ophthalmic conditions.
6.7 Drugs Acting Through Sympathetic Nervous System
Adrenergic agonists and antagonists are the major class of drugs which have been extensively used in ophthalmology. Norepinephrine, epinephrine, and dopamine are the three major adrenergic neurotransmitters of the sympathetic division of the autonomic nervous system. These amines work by activating adrenergic receptors, namely, alpha- and beta-receptors, present in various tissues of the eye which are primarily innervated by postganglionic sympathetic nerves. The distribution of these receptors is mentioned in Table 6.1.
Adrenergic agonists can be classified into directly acting, indirectly acting, and mixed-acting agents. Drugs such as epinephrine which can directly stimulate the receptors by binding to one or more receptors are known as directly acting. Drugs which can increase the release of responsible neurotransmitter from presynaptic vesicles to augment the effect of the sympathetic innervations are known as indirectly acting such as cocaine. Drugs which can perform both the actions of direct and indirect activation are known as mixed acting (Fig. 6.1).
The eye has both alpha- and beta-adrenergic receptors. Adrenergic receptors are present in corneal epithelium, endothelium, iris radial and sphincter muscles, trabecular meshwork, ciliary epithelium, ciliary muscles, lacrimal gland, and retinal pigment epithelium (Table 6.1). The adrenergic system plays a vital role in maintaining the tonicity of this part for the physiological processes of the eye through constriction and dilation. Thus, this system has been a subject for manipulation for various diagnostic, palliative, and curative strategies in ophthalmic conditions.
6.7.1 Directly Acting Sympathomimetics
6.7.1.1 Epinephrine (Alpha 1 Agonist)
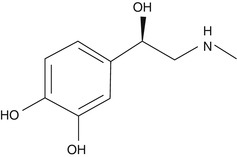
The first hormone ever isolated was epinephrine, which was an iron complex and marketed in 1900 by Farbwerke Hoechst as Suprarenin (Sneader 2001). Epinephrine was also known as adrenaline which is a nonselective but potent stimulant of both alpha- and beta-receptors (α1, α2, α3, β1, and β2). Epinephrine is chiefly secreted through the medulla of the adrenal gland and in the synapses of nerve cells, where it acts as a neurotransmitter (Fig. 6.5a).
Mechanism of Action and Therapeutic Use
Epinephrine binds to alpha- and beta-receptors present in ciliary bodies and the iris. It causes vasoconstriction of the ciliary vessels, resulting in decreased production of aqueous humor through ciliary bodies. It also reduces the aqueous production by ciliary epithelium and facilitates the outflow through trabecular meshwork. This finally reduces the intraocular pressure in the eye. It also causes constriction of the radial muscle of the iris, resulting in miosis. Due to this property and site of action, epinephrine is extensively used in glaucoma and in iritis patients. It is also used before ophthalmic surgery to avoid synechiae.
Dosage
Epinephrine comes in 0.1, 0.5, 1 and 2 % ophthalmic solution for the topical instillation in glaucoma patients. It is recommended to use as a single drop (0.5 or 1 %) 1–2 times daily.
Side Effects
Burning, allergy, reactive hyperemia, headache, and deposition of black oxidation products in the conjunctiva and cornea are the common side effects of topically instilled epinephrine. Angle-closure glaucoma can be induced in the persons who anatomically has narrow iridocorneal angle. A study by Ballin et al. in 1966 reported that the topical ocular use of epinephrine has been shown to be correlated with an increased frequency of cardiac extrasystoles; thus it has to be used cautiously in patients with history of heart disease, hyperthyroidism, or abnormal sensitivity to the systemic effects of epinephrine.
6.7.1.2 Dipivefrin
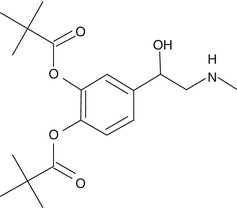
Dipivefrin is an ester analogue and is a prodrug of epinephrine which is formed by the diesterification of pivalic acid and epinephrine. The addition of pivaloyl groups to the epinephrine molecule makes it more lipophilic and hence increases the permeability of this molecule into the anterior chamber of the eye once instilled topically. This drug is widely used to treat open angle glaucoma.
Mechanism of Action and Therapeutic Use
Due to its high lipophilicity, it penetrates into the cornea and there it is hydrolyzed by the esterases to convert into its active constituent of epinephrine. The mechanism of action of dipivefrin is as similar as the parent drug epinephrine. It acts by stimulating α- and/or β2-adrenergic receptors, leading to a decrease in aqueous production and an increase of outflow facility. In comparison to its native molecule epinephrine, dipivefrin is having better penetration, ocular tolerance, and longer-lasting effect. Due to this property, dipivefrin is usually preferred over epinephrine.
Dosage
Dipivefrin is commercially available as 0.1 % ophthalmic solution recommended for once a day use in glaucoma patients, as it is a longer-acting adrenergic receptor agonist.
Side Effects
Side effects include photosensitivity, conjunctival hyperemia, burning, allergy, and hypersensitivity. Caution has to be taken with patients showing hypersensitivity to epinephrine.
6.7.1.3 Phenylephrine
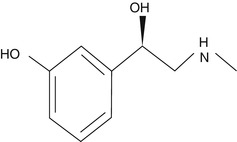
Phenylephrine is a sympathomimetic amine that selectively acts predominantly on alpha-one adrenergic receptors. It is a noncatecholamine and it differs from epinephrine chemically only in lacking hydroxyl group at position 4 on the benzene ring. It activates beta-receptors as well but only at high concentration. So its vasoconstrictor effect is mediated generally through activation of alpha-receptors.
Mechanism of Action and Therapeutic Use
Phenylephrine binds and activates alpha-1 receptors present at the radial muscles of the iris. Activation of alpha-1 receptors at the radial muscles causes radial muscle contraction, resulting in dilation of the pupil (mydriasis). Phenylephrine is a commonly used mydriatic instilled before the examination of the retina.
Dosage
Phenylephrine is used as topical mydriatic ophthalmic solution in the concentration of 0.12, 2.5, and 10 %. This is usually used in conjunction with the tropicamide for the fastest mydriatic effect.
Side Effects
Side effects include hyperemia, burning, allergy, photosensitivity, and hypersensitivity. As it is a potent vasoconstrictor, systemic absorption can cause rise in blood pressure; hence care should be taken with hypertensive patients.
6.7.2 Mixed-Acting Sympathomimetics
6.7.2.1 Ephedrine
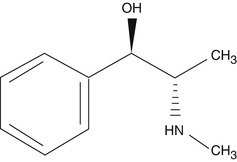
Ephedrine is a mixed-acting sympathomimetic drug which has affinity toward both alpha- and beta-adrenergic receptors. Structurally it is similar to epinephrine, but chemically, ephedrine is an alkaloid which is found in various plants in the genus Ephedra. It mainly enhances the activity of norepinephrine at presynaptic synapses and on adrenergic receptors.
Mechanism of Action and Therapeutic Use
Ephedrine acts on adrenergic receptor alpha and beta postsynaptically by increasing the presynaptic activity of norepinephrine at the presynapses. It causes mydriasis by increasing vasoconstriction effect of norepinephrine on the alpha-receptor present at the iris radial muscle. Study reported that ephedrine is less effective in causing mydriasis in colored individuals than the whites (Obianwu and Rand 1965). It has been speculated by Angenent and Koetle (1953) that the poor mydriatic action of the compound in deeply pigmented human iris may lack enough norepinephrine as the precursor of this neurotransmitter, i.e., dopamine may be diverted to form melanin in the dark iris.
Ephedrine is primarily used as mydriatic for ophthalmic use. Since better options are available, this is not commonly used now.
Side Effects
Tachyphylaxis is the common phenomenon for the ephedrine use.
6.7.3 Indirectly Acting Sympathomimetics
6.7.3.1 Hydroxyamphetamine
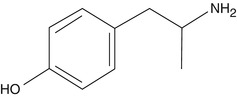
Hydroxyamphetamine is an indirectly acting sympathomimetic drug which is a major metabolite of amphetamine. It is produced after hydroxylation of amphetamine through the CYP2D6 member of cytochrome P450 superfamily.
Mechanism of Action and Therapeutic Use
It acts by decreasing the serotonin and monoamine oxidase metabolism. Inhibition of monoamine oxidases prevents the metabolism of catecholamine in the presynaptic terminal, thus indirectly increasing the amount of norepinephrine at the synaptic cleft. This further antagonizes the action of adrenergic receptors. Adrenergic receptors are present widely in iridial and ciliary bodies. Application of hydroxyamphetamine topically causes mydriasis by increasing the catecholamine at the presynaptic cleft. As this drug does not stimulate the effector cells directly, it has some very important application at diagnosing Horner’s syndrome (oculosympathetic palsy or OSP) in ophthalmic condition. By 1971, hydroxyamphetamine was commercially available in the market for distinguishing between postganglionic and preganglionic or central causes of OSP. Location of lesion in Horner’s disease is diagnosed by instilling the drug topically to induce mydriasis. If the lesion is present preganglionically, then the mydriasis would happen, as postganglionically neurons are intact to release the norepinephrine, whereas postganglionic neuron lesion results in less or no norepinephrine being released, and mydriasis will be incomplete or absent in response to topical hydroxyamphetamine (Smit 2010).
Dosage
Hydroxyamphetamine is commercially available as 1 % ophthalmic solution alone and with conjunction with 0.25 % tropicamide. This ophthalmic preparation is extensively used by the ophthalmologist for the diagnosis and localization of Horner’s syndrome.
Side Effects
The common side effects after instillation include change in color vision, dry mouth, headache, increased sensitivity to light, and burning and stinging sensation in the eye.
6.7.3.2 Cocaine
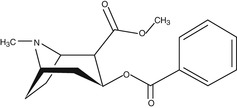
Cocaine is produced from the leaves of the coca plant (Erythroxylum coca). It is an ester of benzoic acid and methylecgonine. It is one of the directly acting sympathomimetics used often for mydriasis and local anesthetic effect.
Mechanism of Action and Therapeutic Use
Cocaine inhibits the reuptake of the neurotransmitter, namely, norepinephrine, dopamine, and serotonin, at postganglionic sympathetic nerve endings. Accumulation of neurotransmitter at the synaptic cleft stimulates the adrenergic receptors. When given topically in the eye, radial muscle of the pupil contracts and causes mydriasis. Cocaine is used from a long time for the diagnosis of anisocoria produced by Horner’s syndrome. Sympathetic innervation disturbance anywhere will result in less or no neurotransmitter release at the synaptic cleft, and cocaine in that case would not be able to produce mydriasis, which is a confirmation tool for the anisocoria that resulted from Horner’s syndrome (Smit 2010). Besides this action, cocaine also inhibits the nerve impulses; thus it is also used as local anesthetic.
Dosage
Cocaine is available in 1–4 % ophthalmic solution for the diagnosis of anisocoria and for local anesthesia prior to ophthalmic surgeries such as lid surgery. Cocaine 2–10 % has been the gold standard in the diagnosis of unilateral Horner’s syndrome for more than 30 years (Smit 2010).
Side Effects
Although no evident side effects have been seen for topically applied cocaine ophthalmic solution, systemic absorption can cause nervousness, excitement, euphoria, confusion, agitation, and restlessness.
6.7.4 Topical Vasoconstrictors (Imidazole Derivatives)
Imidazole derivatives which act as sympathomimetics are usually derived from 2-imidazoline (dihydroimidazoles) which is the isomer of the nitrogen-containing heterocyclic derived from organic compound called imidazole. These imidazoline derivatives are biologically active and have characteristic substituent of aryl or alkyl group between the nitrogen centers. In ocular pharmacology, naphazoline, tetrahydrozoline, and oxymetazoline are being used as topical vasoconstrictors.
6.7.4.1 Naphazoline
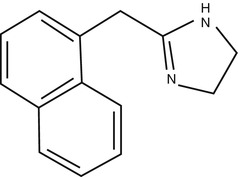
Naphazoline is the derivative of imidazoline, is an alpha adrenergic stimulant, and is used in ophthalmology as a topical vasoconstrictor.
Mechanism of Action and Therapeutic Use
Naphazoline is a sympathomimetic and it causes vasoconstriction by stimulating alpha adrenergic receptors. When applied topically it causes vasoconstriction of the arterioles of the conjunctiva and also produces mydriasis. Naphazoline is used for reducing the conjunctival congestion due to its course of mechanism.
Dosage
Naphazoline is used as ophthalmic decongestant and available in 0.012–0.1 % ophthalmic solutions.
Side Effects
Common ocular side effects of naphazoline include mydriasis, photosensitivity, conjunctival hyperemia, hypersensitivity, lacrimation, and increased intraocular pressure.
6.7.4.2 Tetrahydrozoline
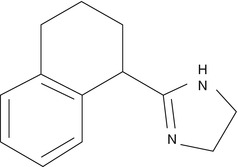
Tetrahydrozoline is also one of the derivatives of imidazoline and is used as conjunctival decongestant in ophthalmology.
Mechanism of Action and Therapeutic Use
Tetrahydrozoline is a sympathomimetic alpha adrenergic agonist which causes vasoconstriction. Tetrahydrozoline is also used as conjunctival decongestant like other imidazoline derivatives. It reduces the congestion by vasoconstricting the arterioles supplying to the conjunctiva.
Dosage
Tetrahydrozoline is available as 0.05 % solution for ophthalmic use for relieving the conjunctival hyperemia. Some dry eye ophthalmic preparation also contains 0.05 % of tetrahydrozoline. It is been advised to use 1–4 times a day according to the congestion status of the conjunctiva.
Side Effects
Tetrahydrozoline has almost similar side effects as other imidazoline derivatives such as burning, photosensitivity, hyperemia, and hypersensitivity.
6.7.4.3 Oxymetazoline
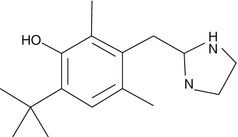
It was developed by Fruhstorfer in 1961 from xylometazoline. Oxymetazoline is a specific alpha-1 and partial alpha-2 agonist. Its specificity toward beta adrenergic receptors is not known.
Mechanism of Action and Therapeutic Use
Oxymetazoline is a sympathomimetic which selectively agonizes alpha-1 receptors and partially agonizes alpha-2 receptors. As vascular capillary beds are rich in alpha-1 receptors, after topical instillation, it acts on endothelial postsynaptic adrenergic receptors and causes vasoconstriction.
Usage
Oxymetazoline is available in 0.025 % of ophthalmic solution which is prescribed four times a day. As rebound hyperemia is one of the common side effects of this drug, it is not advised to be used for more than 72 h.
Side Effects
Oxymetazoline can cause severe burning, stinging, pain, and eye irritation. As it is specifically agonizes alpha-1 receptors in blood vessels, if absorbed systemically it can cause high blood pressure and tachycardia.
6.7.5 Alpha-2 Adrenergic Receptor Agonist
The α(2)-adrenergic receptors are predominantly present at the ciliary epithelium where aqueous humor production takes place. Involvement of these receptors is found to have an enormous importance in the treatment of ocular hypertension, glaucoma, uveitis, strabismus, and several diagnostic purposes.
6.7.5.1 Brimonidine
Brimonidine is used as a tartrate salt and it is a selective α(2)-adrenergic receptor agonist with potent vasoconstrictor activity. It is approved for the treatment of open-angle closure glaucoma and ocular hypertension. It has been found to have a neuroprotective role in the retinal ganglionic cells to prevent impairment of vision (Vidal-Sanz et al. 2001).
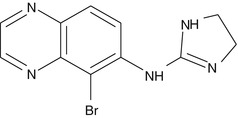

Mechanism of Action
Brimonidine activates membrane-bound G-protein-coupled receptors to inhibit adenylate cyclase which in turn reduces cAMP. Thus, it leads to constriction of smooth muscle and blood vessels. The primary site of action is the ciliary epithelium where brimonidine stimulates the receptor, in turn reducing the rate of aqueous humor production at the ciliary epithelium and at the same time increasing the uveoscleral outflow (Toris et al. 1995a). It is highly selective on α(2)-adrenergic receptor and accounts for 7–12-fold and 23–32-fold more than clonidine and apraclonidine respectively.
Therapeutic Uses
It is the drug of choice for the treatment of chronic open-angle glaucoma and ocular hypertension. It is considered as a major therapy in patients who are contraindicated to beta-blockers (Apatachioae and Chiseliţa 1999). Brimonidine (0.2 %) with brinzolamide (1 %) in fixed combination resulted in significantly greater IOP-lowering effect when compared to brimonidine or brinzolamide alone and exhibited a good safety profile (Aung et al. 2007).
It is commercially available as an ophthalmic solution at 0.15 %w/v and 0.2 %w/v. The topical solution is indicated to be instilled three times a day every 8 h apart. The peak effect of lowering ocular hypotension is observed after 2 h post dosing.
Side Effects
It has low incidences of ocular and peripheral side effects. Photosensitivity, ocular hyperemia, and hypersensitivity are the major side effects. Long-term use has been reported to cause anterior uveitis and allergic conjunctivitis (Becker et al. 2004). It is contraindicated in infants and children due to severe systemic side effects (Wright and Freedman 2009).
6.7.5.2 Apraclonidine
Apraclonidine, a para-amino derivative of clonidine, has shown low penetration across the blood-brain barrier, thus minimizing cardiovascular and systemic side effects. It stimulates α(2)-adrenergic receptors present on nonpigmented ciliary epithelium which in turn leads to reduction of IOP (Gharagozloo et al. 1988).
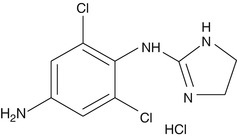

Mechanism of Action
Apraclonidine is a relatively selective agonist of prejunctional α(2)-adrenergic receptors present on nonpigmented ciliary epithelium. It causes inhibition of membrane-bound adenylate cyclase which in turn impairs the conversion of ATP to c-AMP, thereby reducing production of aqueous humor (Gharagozloo et al. 1988). Vasoconstriction of arterioles also resulted in less ciliary body blood flow, thus reducing aqueous production. It also influences the aqueous humor drainage pathway which includes mostly the uveoscleral outflow, and in less instances, conjunctival and episcleral vascular flow may also be involved (Hurvitz et al. 1991; Toris et al. 1995b).
Therapeutic Uses
Apraclonidine is approved for the treatment of elevated IOP in normotensive and glaucomatous human eyes by suppressing the rate of aqueous production (Yüksel et al. 1992). In a study, apraclonidine at 0.5 % was found to have better therapeutic efficacy than 1 % ophthalmic solution in reducing IOP (Rosenberg et al. 1995). It has been found useful as a short-term adjunctive therapy to timolol for the poorly controlled glaucoma (Morrison and Robin 1989; Stewart et al. 1995). It has been used as a diagnostic agent for Horner’s syndrome (Chen et al. 2006). It has been reported that it is safe and effective as a single postoperative administration for reducing the elevated IOP after argon laser trabeculoplasty (Holmwood et al. 1992; Robin et al. 1987b), argon laser iridotomy (Hong et al. 1991; Robin et al. 1987a), and Nd-YAG laser capsulotomy (Brown et al. 1988; Cullon and Schwartz 1993; Pollack et al. 1988).
Dosage
Apraclonidine is available commercially at 0.5 and 1 %w/v ophthalmic solution. It needs to be instilled one drop three times daily. Within 1 h post instillation, it produces 20 % reduction in IOP in the glaucomatous eye and maximum effect can be seen at 3–5 h. Its duration of action for IOP reduction stays for 5–8 h. It is commonly administered as an adjunctive therapy to timolol for reducing IOP. It is contraindicated in patients receiving monoamine oxidase (MAO) inhibitors or tricyclic antidepressants.
Side Effects
Ocular side effects include photosensitivity, transient lid retraction, mydriasis, and conjunctival blanching. Transitory Snellen acuity and allergic conjunctivitis are the other side effects.
6.7.6 β-Blockers
This class of drugs has become a major therapeutic option for the treatment of glaucoma and ocular hypertension. Due to their well tolerance and minimal side effects, β-blockers are the mainstay for the management of glaucoma classified as nonselective and selective β-blockers.
6.7.6.1 β-Blockers (Nonselective)
Timolol
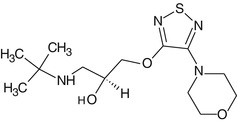
Timolol is a potent and standard nonselective β-adrenergic receptor blocker. It was the first beta-blocker introduced in 1978 for clinical use in ophthalmology after confirming its efficacy as an ocular hypotensive agent due to blocking effect at β(1)- and β(2)-adrenergic receptors. Currently it is a prototype drug against which all newly developed β-blockers are compared for the reduction in IOP in clinical trials of glaucoma. It does not have any significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic (membrane-stabilizing) activity but does possess a relatively high degree of lipid solubility.
Mechanism of Action
Aqueous humor production governs by the stimulation of β-adrenergic receptor-mediated c-AMP-PKA pathway. Timolol acts by blocking both β(1)- and β(2)-adrenergic receptors present at ciliary processes and epithelium, resulting into a decrease in intracellular c-AMP, thus reducing aqueous humor formation. It does not inhibit the active transport system or prostaglandin biosynthesis. It also decreases ocular blood flow which in turn leads to a decrease in ultrafiltration responsible for aqueous production (Zimmerman and Kaufman 1977; Neufeld et al. 1983). It does not have any effect on aqueous outflow.
Therapeutic Uses
Timolol is used as a first-line agent found effective in reducing IOP (both on immediate and long-term basis) in ocular hypertension and glaucoma. It is effective in reducing IOP when compared to pilocarpine and epinephrine alone (Boger et al. 1978; Moss et al. 1978). Topical timolol when given in patients receiving oral beta-blockers for hypertension further reduces IOP (Maren et al. 1982). It has an additive effect when added to treatment regimen of miotics and carbonic anhydrase inhibitors (Boger et al. 1978; Sonty and Schwartz 1979).
Dosage
Timolol is available commercially at 0.25 and 0.5 % ophthalmic solution. It should be instilled one drop in each eye two times daily. The duration of action following topical instillation of a single drop persists at least 24 h. Its action is dose dependent and it has been shown that 0.5 % concentration gives maximum reduction in IOP.
Side Effects
Timolol is well tolerated with mild stinging or burning sensation, conjunctival hyperemia, and blurred vision.
Levobunolol
Levobunolol is a nonselective beta-adrenergic receptor antagonist. It was introduced as an alternative to timolol for the better management of glaucoma and ocular hypertension. Levobunolol has claimed to be as effective as timolol but having longer duration of action, resulting in better patient compliance after single topical application.
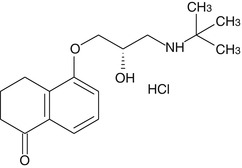

Mechanism of Action
Levobunolol shows the same mechanism of action as that of timolol. It suppresses aqueous humor production by blocking β(1)- and β(2)-adrenergic receptors. It also reduces the ocular blood flow due to blocking of β-receptor-mediated dilation of ciliary vessels. It does not have any significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic (membrane-stabilizing) activity.
Therapeutic Uses
It is used in both short-term and long-term management of open-angle glaucoma and ocular hypertension. Its longer duration of action would allow single topical dosing per day (Rakofsky et al. 1989; Silverstone et al. 1991; Wandel et al. 1986). Its action on reducing IOP and ocular hypertension is roughly equivalent to that of timolol (Bensinger et al. 1985; Boozman et al. 1988; Silverstone et al. 1991).
Dosage
Levobunolol is available commercially as 0.5 % ophthalmic solution. It needs to be instilled one drop once a day in each eye. The peak reduction in IOP was found to occur within 1 h after single topical application. The duration of action following topical instillation of a single drop persists at least 24 h.
Side Effects
Mild stinging or burning sensation is noted after topical instillation.
Metipranolol
Metipranolol is a nonselective β-adrenoceptor blocker structurally similar to timolol, propranolol, and levobunolol. It is well tolerated and does not have any significant intrinsic sympathomimetic activities, direct myocardial depressant, or local anesthetic (membrane-stabilizing) activity.
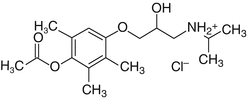

Mechanism of Action
Metipranolol reduces IOP in the glaucomatous eye and normal eye by the same mechanism of action as that of timolol. It suppresses aqueous humor production by blocking β(1)- and β(2)-adrenergic receptors. It also reduces the ocular blood flow due to blocking of β-receptor-mediated dilation of ciliary vessels. It does not affect aqueous outflow (Battershill and Sorkin 1988).
Therapeutic Uses
It is used in the treatment of open-angle glaucoma and ocular hypertension. Clinical significance of metipranolol with 0.1–0.5 % in glaucoma was found to have comparable to timolol, 0.25–0.5 % (Merte et al. 1983; Mills and Wright 1986), and levobunolol, 0.5–1 % (Krieglstein et al. 1987; Stryz and Merte 1985). Twice-daily administration of metipranolol (0.1 %) was found to be effective when compared to timolol 0.25 % and was associated with fewer side effects at lower concentration (Schmitz-Valckenberg et al. 1984).
Dosage
Metipranolol is available commercially as 0.3 % ophthalmic solution. It needs to be administered one drop two times a. The peak reduction in IOP was observed at 2 h. The duration of action after topical instillation persists for at least 24 h.
Side Effects
Mild stinging or burning sensation is noted after instillation. Bronchoconstriction, CNS effects and effect on blood pressure and heart rate have been also observed. It is contraindicated in patients with bradycardia, heart block, heart failure, chronic asthma, and other respiratory disorders. Its use in glaucoma is associated with development of granulomatous uveitis (Melles and Wong 1994; Akingbehin and Villada 1991) which further leads to increase in IOP.
Carteolol
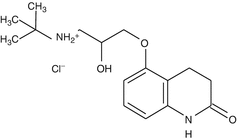
Carteolol is a nonselective β-adrenergic receptor antagonist with intrinsic sympathomimetic activity. Thus, this agent can be used safely in cardiac and asthmatic patients without precipitating systemic side effects such as bradycardia and bronchospasm, respectively (Henness et al. 2007). This drug is structurally similar to timolol and levobunolol. It is the second most topical drug prescribed worldwide in glaucoma.
Mechanism of Action
Carteolol shows same mechanism of action as that of timolol. It suppresses aqueous humor production by blocking β(1)- and β(2)-adrenergic receptors. It also reduces the ocular blood flow due to blocking of β-receptor-mediated dilation of ciliary vessels.
Therapeutic Uses
It is approved for the treatment of open-angle glaucoma and ocular hypertension. It possesses intrinsic sympathomimetic activity that produces minimal adrenergic agonistic activity which in turn causes fewer systemic side effects in patients suffering with cardiac and pulmonary diseases (Steward et al. 1991). In a crossover study, no significant difference was found in reduction of IOP in the glaucomatous eye between carteolol 1 and 2 % ophthalmic solutions (Duff 1987). In another study, carteolol 1 and 2 % topical solution administered twice daily for a month was compared with timolol 0.5 % and metipranolol 0.3 % and showed similar efficacy in reducing IOP in human patients (Scoville et al. 1988; Steward et al. 1991; Mirza et al. 2001). Carteolol has offered protection on visual function in addition to the treatment of glaucoma due to intrinsic sympathetic activity which reduces peripheral vascular resistance in ciliary vessels, thus improving perfusion of the optic nerve head measured by color Doppler imaging (Montanari et al. 2001).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


