Obstructive Pulmonary Disorders
Lorna L. Schumann
Key Questions
• What are the clinical manifestations and common causes of acute airway obstruction?
• What is the role of inflammation in the development of asthma?
• What pulmonary function test abnormalities are characteristic of obstructive pulmonary disorders?
![]()
http://evolve.elsevier.com/Copstead/
Obstructive lung diseases are manifested by increased resistance to airflow. Obstructive diseases of the lung can be classified into those involving (1) obstruction from conditions in the wall of the lumen (e.g., asthma, bronchitis), (2) obstruction resulting from increasing pressure around the outside of the airway lumen (e.g., emphysema secondary to loss of lung tissue and elasticity, enlarged lymph node, or tumor), and (3) obstruction of the airway lumen (e.g., presence of a foreign body, excessive secretions, aspiration of fluids).1,2
These classifications are mainly terms of convenience, because many respiratory disease processes involve several areas of the pulmonary system. Involvement of the airways produces narrowing of the passages so that airflow obstruction occurs. The major obstructive airway diseases are asthma, bronchitis, and emphysema.
Obstruction from Conditions in the Wall of the Lumen
Asthma
Etiology
Asthma is a lung disease characterized by (a) airway obstruction that is reversible (but not completely in some patients); (b) airway inflammation; and (c) increased airway reactivity to a variety of stimuli.2,3 In terms of symptoms, asthma is defined by paroxysms of diffuse wheezing, dyspnea, and cough, resulting from spasmodic contractions of the bronchi. Airway inflammation leads to epithelial denudation, collagen deposition beneath the basement membrane, mast cell activation, mucosal edema, increased viscid secretions, and smooth muscle contraction. With proper treatment, most patients with asthma can control the disease and prevent development of emphysema or bronchitis. Asthma occurs in about 5% to 12% of the U.S. population and is common among children and adults.2–6 Asthma is the most common chronic disease of childhood.6,7 High-risk populations include African Americans, inner-city dwellers, and premature or low-birth-weight children.5,7 The pathophysiology of both intrinsic (non-allergic, sometimes referred to as adult onset) and extrinsic (allergic, sometimes referred to as pediatric onset) asthma is thought to involve inflammation of the airways. Most cases of asthma can be triggered both by allergens and by stimuli, such as exercise and exposure to cold air. The terms intrinsic and extrinsic are still used, but many prefer the terms non-allergic and allergic. The clinical features of all forms are similar.
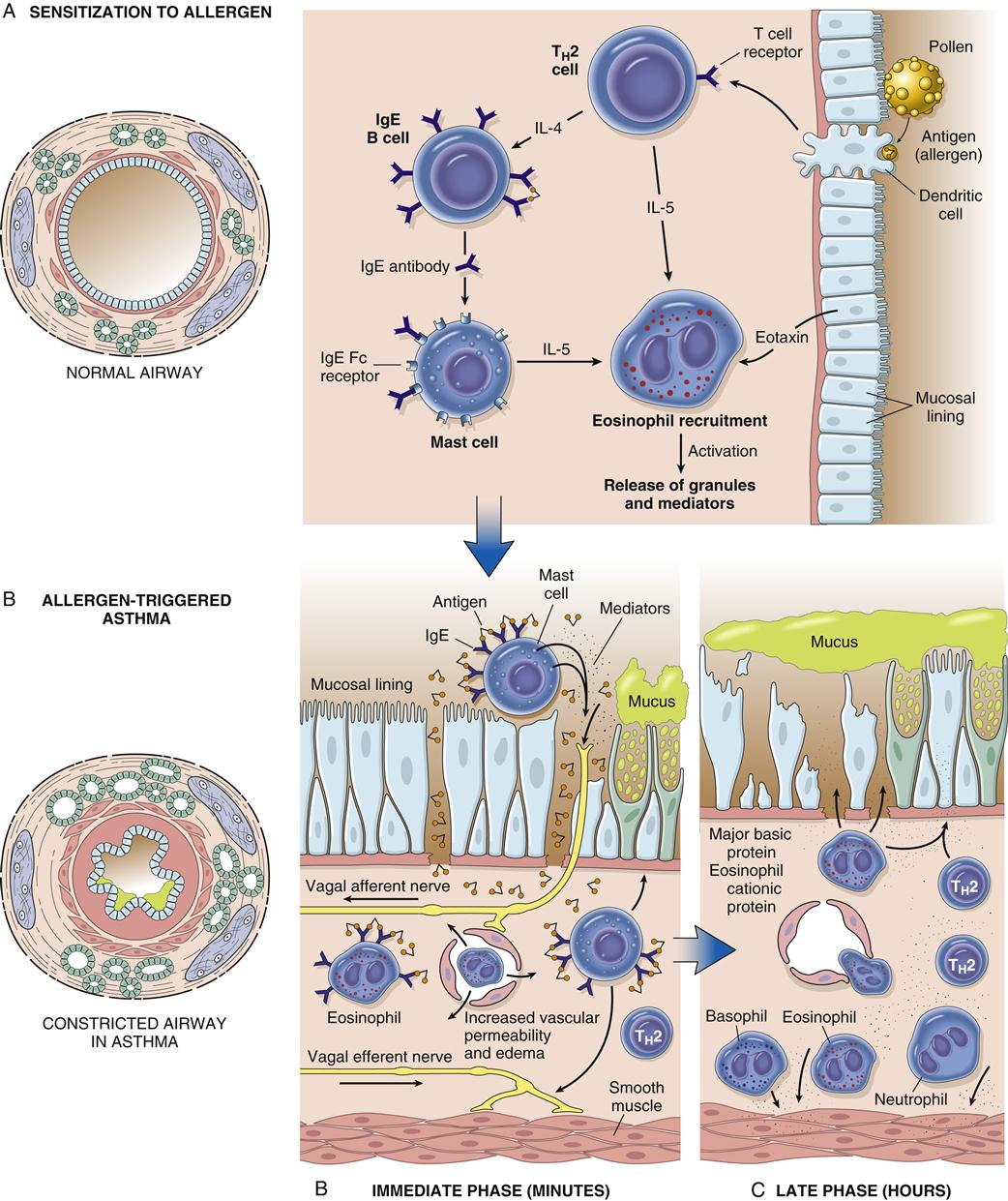
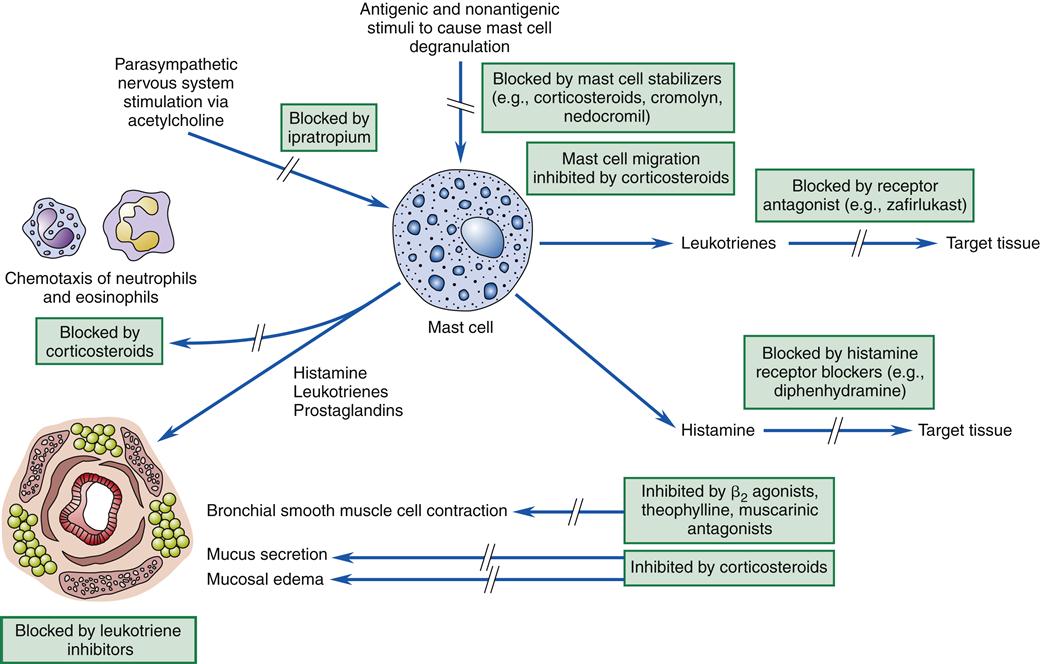
Asthma is associated with the release of inflammatory chemicals from mast cells in the airways. The mechanisms stimulating mast cell release are allergic, immunoglobulin E (IgE)-mediated triggers for extrinsic/allergic asthma (Figure 22-1). Intrinsic/non-allergic asthma occurs in patients who have no history of allergy.8 Allergic asthma (extrinsic) comprises approximately one third to one half of all cases.8,9 Asthma is often associated with a history of hay fever or eczema (atopy), a positive family history of the disease, and positive skin test reactions to allergens (dust mites, cat/dog dander, industrial chemicals).8,10 Pharmacologic therapy, allergen-specific immunotherapy, and environmental control are usually beneficial.6,9 Refer to Chapter 10 for details about IgE-mediated mechanisms and hyposensitization methods.
Intrinsic/non-allergic asthma frequently develops in middle age and has a less favorable prognosis. Respiratory tract infections or psychological factors appear to be contributory, whereas antigen-antibody reactions appear to have less of a role in the disease process, although IgE levels may be elevated.8–10 Attacks are often severe, and patients have a variable response to medical therapy.10 Allergen-specific immunotherapy and environmental control measures are not usually helpful. Airways are hyperreactive, and patients may present with extreme dyspnea, orthopnea, and agitation.
Exercise-induced asthma is common, especially in children and adolescents.8,9 Bronchospasm often occurs within 3 minutes after the end of exercise and usually resolves in 60 minutes.9,10 Heat loss, water loss, and increased osmolarity of the lower respiratory mucosa are believed to stimulate mediator release from basophils and tissue mast cells. This mediator release produces airway smooth muscle contraction. Running, jogging, and playing tennis are the most common instigators of exercise-induced asthma. Bicycling and swimming are much less likely to induce symptoms.
Occupational asthma may be accompanied by positive skin test reactions to protein allergens in the work environment. Occupational exposures to allergens, such as fumes from plastic, formaldehyde, isocyanates, some metals, textiles, engine exhaust, sulfur dioxide, fluoride, and western red cedar dust, do not provoke skin reactions.10,11 To prove hypersensitivity, it may be necessary to conduct challenge tests in the patient by inhalation of the suspected dust or fumes in a controlled environment. The individual affected by occupational asthma tends to have progressively more severe attacks with subsequent exposures. Symptoms may clear over a weekend or vacation and recur when the individual returns to the work environment.10 This repeated history often is sufficient to establish the diagnosis. Hyposensitization in most cases of occupational asthma is ineffective because of lack of an IgE antibody reaction and because the chemicals that cause symptoms usually are toxic when injected.10,11
Drug-induced asthma can produce symptoms ranging from mild rhinorrhea to respiratory arrest requiring mechanical ventilation. In patients with nasal polyps, sinusitis, and asthma, ingestion of aspirin may induce severe or occasionally fatal asthmatic attacks. Sometimes anaphylactoid reactions cause a decrease in blood pressure, itching (pruritus), rhinorrhea, or a rash after aspirin ingestion. Aspirin intolerance with asthma usually occurs in adults. Attacks may occur within minutes of ingestion or may be delayed up to 12 hours. Nonsteroidal antiinflammatory drugs such as indomethacin (Indocin), ibuprofen (Motrin, Advil), and related drugs may also induce asthma in the aspirin-intolerant patient. Aspirin reactions are not immunologically mediated. Therefore, skin testing is not useful for diagnosing aspirin intolerance. Because aspirin and nonsteroidal antiinflammatory drugs inhibit the conversion of arachidonic acid to prostaglandins, it is possible that aspirin shunts arachidonic acid breakdown products to the leukotriene system. Leukotrienes, released from mast cells, are slow-reacting substances of anaphylaxis with powerful bronchoconstriction activity (see Figure 22-1). Avoidance is the most practical approach to this problem because testing can be dangerous.
Asthma can occur from ingestion of food additives. Tartrazine (yellow dye no. 5), which is used to color pharmaceuticals, hair products, and food products, may also produce severe asthma in susceptible persons. A complete list of drugs containing tartrazine can be obtained from the Food and Drug Administration.
Monosodium glutamate (MSG), used as a flavor enhancer in foods, can produce faintness, nausea, sweating, a fall in blood pressure, and, occasionally, asthma. Sodium or potassium metabisulfite, used to preserve fruits, vegetables, and meats, can cause anaphylactoid reactions. A challenge with the chemical may be necessary to establish a diagnosis, as metabisulfites are widespread in our society.
Hops in beer have also been implicated in causing severe bronchospasm. Skin reactivity does not occur, and the mechanism of the problem is not IgE mediated. The diagnosis involves a history of exposure followed by symptoms.
Pathogenesis
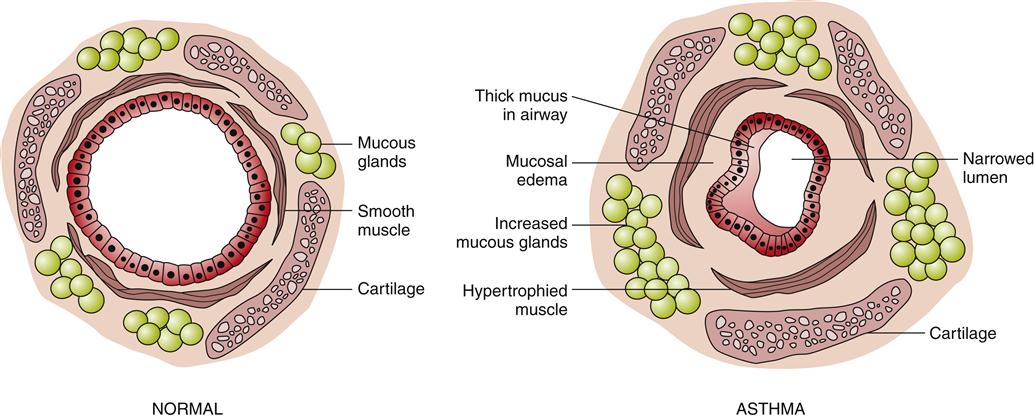
The immunohistopathologic features of asthma include denudation of airway epithelium, collagen deposition beneath the basement membrane, edema, mast cell activation, and inflammatory cell infiltration by neutrophils, eosinophils, and lymphocytes.1,3 Inflammation of the airway contributes to acute bronchospasm (bronchoconstriction), mucosal edema, mucous plug formation, and airway wall remodeling (Figure 22-2). Genetic predisposition (chromosomes 5, 11, 14) for atopy and structural predisposition (smaller airways) are the strongest predisposing factors for developing asthma.3,5 There is a strong association of the ADAM33 gene with asthmatic bronchial hyperresponsiveness.8
With allergic asthma, an IgE-mediated response is common and is manifested by elevated IgE levels, allergic rhinitis, eczema, a positive family history of allergy, and attacks associated with seasonal, environmental, or occupational exposure. The mechanism of action is initiated by exposure to a specific antigen that has previously sensitized mast cells in airway mucosa. When the antigen reacts with the antibody on the surface of the mast cell, packets of chemical mediator substances stored in the cell are released. The chemical mediators that are released include histamine, slow-reacting substances of anaphylaxis (leukotrienes), prostaglandins, bradykinins, eosinophilic chemotactic factor, serotonin, and others.1–3,7 Figure 22-1 depicts common chemicals that are released by the mast cell and the physiologic effect of these chemicals. Cytokines are probably the most important inflammatory mediators, particularly those associated with TH2 helper T cell activation (granulocyte-macrophage colony–stimulating factor and interleukins 3, 4, 5, and 13). These cytokines may be responsible for modulating inflammatory and immune cell function.1,2 Other inflammatory mediators are arachidonic acid metabolites such as leukotrienes and prostaglandins, platelet activating factor (PAF), neuropeptides, reactive oxygen species, histamine, and adenosine. With the release of chemical mediators, the normal respiratory epithelium is denuded and replaced by goblet cells, resulting in mucosal edema, production of inflammatory exudates, and hyperresponsiveness of the airway (bronchoconstriction and leakage).2,12,13 Alterations in epithelial integrity lead to increased microvascular permeability. A secondary mediator response occurs 6 to 12 hours after the primary asthma attack and is more refractory to treatment. Neutrophil chemotactic factor may be the cause of this secondary response.10
Histologic changes in the epithelial basement membrane occur over time. The basement membrane is a complex structure that separates endothelial cells from underlying stroma. The membrane provides tensile strength and physical support to surrounding structures.8 It also functions as a filter and as a site for cell attachment. In a classic study by Hogg in 1982, the width of the basement membrane was shown to thicken in asthmatic patients over time.12 The width seen in asthmatic patients is 17.5 μm, whereas that seen in healthy subjects is 7 μm. Airway remodeling has been detected pathologically. Declines in pulmonary function over time can progress to chronic obstructive pulmonary disease (COPD).10 Figure 22-1 depicts the pathogenesis of asthma in relation to mast cell release and parasympathetic stimulation by way of the vagus nerve. Vagal stimulation leads to edema, mucus hypersecretion, and bronchoconstriction. The nerve endings of asthmatic patients have been found to be devoid of the bronchodilator neuropeptide vasoactive intestinal peptide.10
Clinical manifestations
Common symptoms are wheezing, feelings of tightness of the chest, dyspnea, cough, and increased sputum production.10 Some patients have only a chronic dry cough, and others have a productive cough.9 Especially in children, cough is often the earliest sign of exacerbation of asthma. Wheezing is caused by vibration in narrowed airways, which act like the vibrating reed of a wind instrument, yielding a musical sound.14 Because airways naturally widen with inspiration, inspiratory wheezes reflect increased constriction. Sputum is often thick, tenacious, scant, and viscid (sticky). Physical findings vary with the severity of the attack. A mild attack may be associated with a random monophonic expiratory wheezing associated with airway narrowing, tachycardia, and tachypnea. Random monophonic wheezes are located throughout the chest and are intermittent on examination. The area in which the wheezes are heard best is indicative of the area of obstruction (e.g., if they are heard best at the mouth, this is indicative of large airway obstruction).14,15 Tachycardia is an early sign of hypoxemia. A more severe attack requiring medical assistance may be accompanied by the use of accessory muscles of respiration, intercostal retractions, distant breath sounds with inspiratory wheezing, orthopnea, agitation, tachypnea, and tachycardia. In the severe state, the patient may appear cyanotic, agitated, restless, and confused. The intensity of wheezing is not a reliable indicator of blockage of airflow. The measurement of peak expiratory flow rate (PEFR) is the best indicator of reduction in airflow (see discussion under Diagnosis). PEFRs are affected by weight, height, age, gender, ethnicity, posture, effort, smoking, and circadian rhythm. A PEFR of less than 80 L/min indicates severe obstruction.9,16 When obstruction is the tightest, the patient cannot move enough air with enough velocity to make wheezing sounds. Isolated inspiratory wheezing may be an indicator of large airway obstruction caused by mucus or laryngeal obstruction.4 A patient with severe respiratory distress, prolonged expiration (indicating that the person is having difficulty moving air out of the lungs), neck and intercostal retractions, and minimal air sounds is critically ill and requires emergency intervention.
Diagnosis
The diagnosis of asthma is based on history, physical findings, sputum examination, pulmonary function tests, blood gas analysis, and chest radiography. Radiographic findings may be normal or may show evidence of hyperinflation with flattening of the diaphragm in progressive disease.8 Abnormal physical findings include cough, wheezing, a hyperinflated chest, and decreased breath sounds. Asthmatic sputum samples reveal Charcot-Leyden crystals (formed from crystallized enzymes from eosinophilic membranes), eosinophils, and Curschmann spirals (mucous casts of bronchioles).
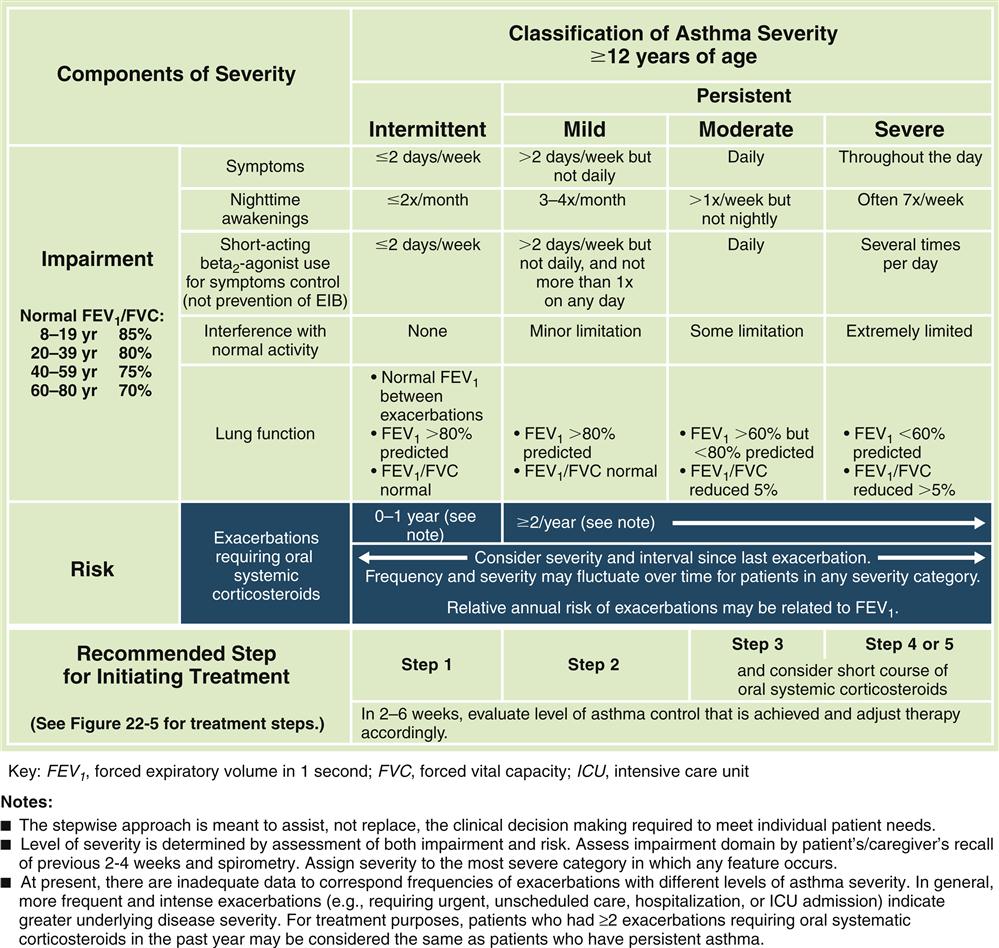
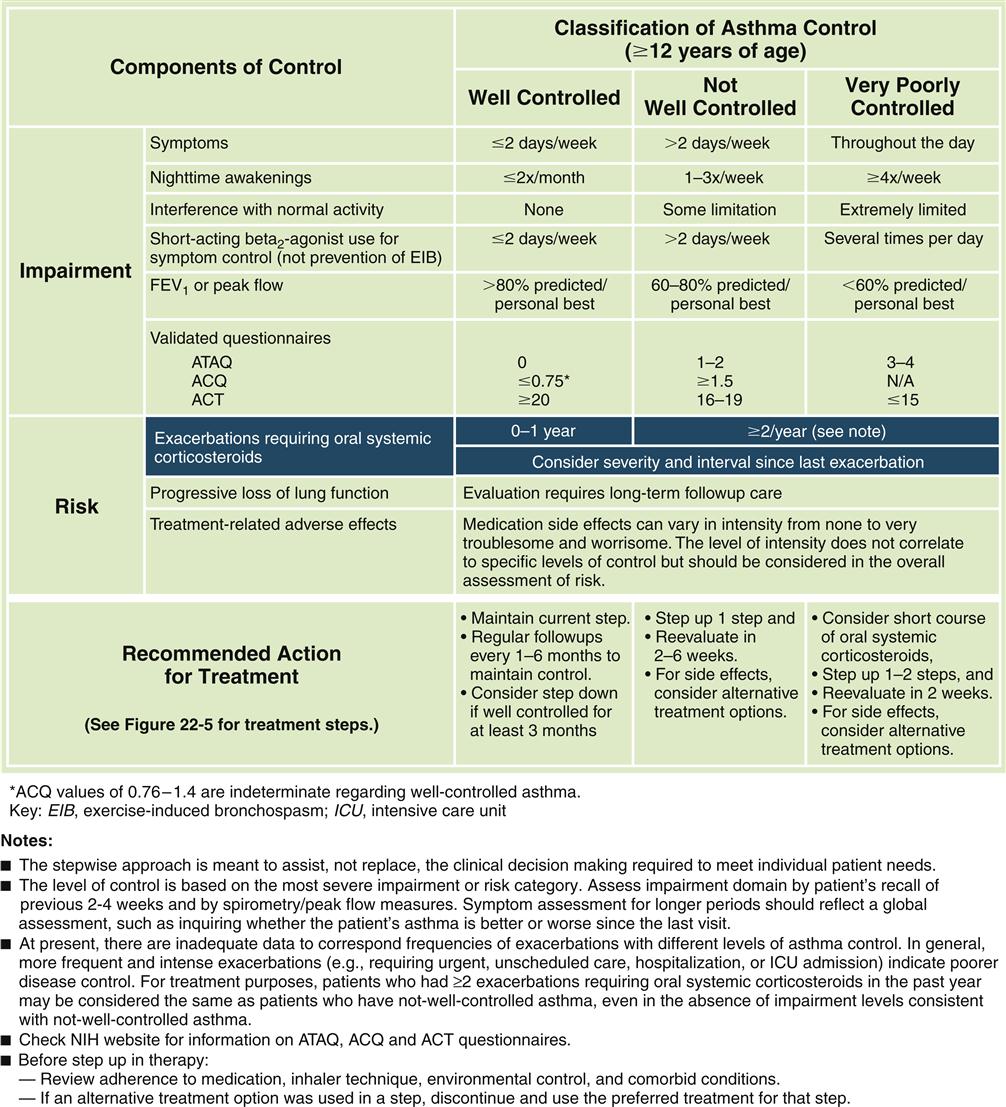
Forced expiratory volumes decrease during asthma attacks. PEFR is measured to determine the index of airway function. The PEFR is the maximal flow of expired air attained during a forced vital capacity (FVC) procedure.15 The evaluation of asthma should include the measurement of forced expiratory volume over 1 second (FEV1), FVC, and the FEV1/FVC ratio before and after administration of a short-acting bronchodilator.8 Airflow obstruction is indicated by a FEV1/FVC ratio of less than 75%.9 Classification of asthma severity and control (Figures 22-3 and 22-4) is based on presenting symptoms, frequency of nighttime symptoms, and lung function.2 Figure 22-5 shows stepped therapy for asthma based on classification.
Arterial blood gas values may be normal during a mild attack, but as the bronchospasm increases in intensity, respiratory alkalosis and hypoxemia become prominent findings. Elevation of arterial partial pressure of carbon dioxide (Paco2) is a poor prognostic sign, indicating that the patient’s ability to continue breathing at a rapid rate has diminished and that exhaustion is imminent.
Respiratory failure may be manifested by severe respiratory distress in a patient who shows no radiographic evidence of pneumothorax. As the patient improves, the wheezing becomes louder. When wheezing is no longer heard after an asthma attack, pulmonary function tests may continue to show obstructive changes for several weeks. Some patients have a slight monophonic wheeze continuously between asthma bouts and still are comfortable and functional.14
Determination of allergens is done by skin testing or inhalation of suspected allergens. Skin testing is usually more helpful in young patients who have extrinsic asthma. Bronchial provocation testing with histamine or methacholine9 may be useful in confirming the diagnosis of asthma in certain cases (see the Diagnostic Tests section later in the chapter).
A complete blood cell count can show an elevated number of white blood cells (WBCs) with an increased number of eosinophils. Eosinophils are prominent in the cellular infiltrate of the bronchioles, the sputum, and the peripheral blood. A decline in the total eosinophil count is a valuable measure of effectiveness of corticosteroid treatment. With effective treatment, the total eosinophil count is depressed below 10/μL.1,9
Treatment
Patients should be taught to avoid the objects in the environment that trigger asthma attacks. Environmental control includes control of dust; removal of allergens such as feathers, molds, and animal dander; and, in some cases, removal of rugs and carpets. Other environmental control factors that help some patients include the use of air purifiers and air conditioners. The patient should also be taught preventive therapy in regard to smoking cessation and avoidance of passive smoke, aerosols, and odors. Patients should seek early treatment for respiratory tract infections.17
Pharmacologic therapy for all three major obstructive disorders is similar and focuses on decreasing inflammation and bronchoconstriction, including β2 agonists, corticosteroids, leukotriene modifiers, and mast cell inhibitors (Figure 22-6). Other therapies used in patients with more severe asthma include home oxygen therapy and home administration of small-volume nebulizer treatments via intermittent positive-pressure ventilation. At home, peak flow monitoring is helpful to parents or patients in determining a treatment plan and when to seek medical assistance. Peak flow meters are also helpful in monitoring progress of the patient with around-the-clock therapy.10
Allergen-specific immunotherapy (hyposensitization) may be used as an adjunct to other therapies. The allergen is first identified by testing with purified allergens using the scratch, prick, or intradermal method. Desensitization therapy has been shown in controlled studies to reduce the frequency and severity of asthmatic episodes when a single offending allergen can be identified.10
Status asthmaticus (severe attack unresponsive to routine therapy) requires more rapid and intense therapy, which may include epinephrine, subcutaneous terbutaline, and/or aminophylline. Once airflow has improved, aerosol bronchodilating inhalers may be used. Intravenous corticosteroids are the mainstay of therapy. Oxygen therapy with or without mechanical ventilation may be necessary in severe cases.
The more patients understand about their asthma, the better they are at self-managing their symptoms. Educational materials are available from the American Lung Association, the Asthma and Allergy Foundation of America, and the National Institute of Allergy and Infectious Diseases.
Acute Bronchitis
Etiology
Acute inflammation of the trachea and bronchi is produced most commonly (80% of the 12 million cases per year in the United States) by a variety of viruses such as influenza virus A or B, parainfluenza virus, respiratory syncytial virus, coronavirus, rhinovirus, Coxsackie virus, and adenovirus. Nonviral causes include Streptococcus pneumoniae, Haemophilus influenzae, mycoplasma, moraxella, and Chlamydia pneumoniae.7,8 Numerous other pathogens as well as heat, smoke inhalation, inhalation of irritant chemicals (e.g., sulfur dioxide or chlorine, bromine, or fluorine gases), and allergic reactions have also been identified.18 Highest incidences are noted in smokers, young children, and the elderly, with a prevalence in the winter months.8,19 The swelling of bronchial mucosa in children associated with obstruction, respiratory distress, and wheezing is known as asthmatic bronchitis. Acute bronchitis differs from bronchiolitis in the size of the airways affected (i.e., trachea and bronchi as opposed to the small bronchioli).20
Pathogenesis
The airways become inflamed and narrowed from capillary dilation, swelling from exudation of fluid, infiltration with inflammatory cells, increased mucus production, loss of ciliary function, and loss of portions of the ciliated epithelium. Many viruses and mycoplasmal bacteria inhibit macrophages and lymphocytes, temporarily promoting secondary bacterial invasion. Microorganisms may also induce long-lasting hyperirritability of the respiratory tract with associated episodes of bronchospasm.
Clinical manifestations
The presentation of acute bronchitis is usually mild and self-limited, requiring only supportive treatment. Cough may be productive or nonproductive. Associated symptoms include low-grade fever, substernal chest discomfort, sore throat, postnasal drip, and fatigue. In children, the smaller airways are easily obstructed by inflammation, so that severe obstruction may occur.8 The smallness of airways in proportion to body size is due to a smaller lumen in relation to the vessel wall. Associated inflammation of the larynx and trachea produces croup (see Croup Syndrome section in this chapter for further details).
Diagnosis
Diagnosis of acute bronchitis is usually based on the clinical presentation, with recent onset of cough being the distinctive hallmark. Neither the appearance of purulent sputum nor the determination of an increased WBC count is a reliable diagnostic indicator. A chest radiograph is required to distinguish acute bronchitis (normal radiograph) from pneumonia (pulmonary infiltrates on radiograph).
Treatment
Acute bronchitis is predominantly caused by viruses (rhinovirus, coronavirus, adenovirus, influenza virus). Viral infections do not respond to antimicrobial therapy, and symptoms resolve spontaneously in most normal, otherwise healthy individuals. Acute bronchitis caused by bacterial organisms responds well to antibiotic therapy. Codeine-containing medications are helpful in relieving the cough associated with bronchitis that interferes with sleep. Nonpharmacologic recommendations are to increase fluid intake, avoid smoke, and use a vaporizer in the bedroom.
The dangers of acute bronchitis include the potential for bacterial invasion, which can worsen symptoms in patients with chronic obstructive pulmonary disease (COPD) and precipitate serious infections in elderly patients or those with debilitating disease.
Chronic Bronchitis
Etiology
The next two sections of this chapter present chronic bronchitis and emphysema. Characteristic pathologic and clinical findings are described for each of these classifications. Clinically, pure forms of emphysema and chronic bronchitis are rare, and most patients present a combination of both of these obstructive processes. Patients with emphysema and chronic bronchitis constitute most cases of COPD.
The major causes of chronic bronchitis are cigarette smoking (90% of cases),12 repeated airway infections, genetic predisposition, and inhalation of physical or chemical irritants.8,9,21,22
Chronic bronchitis (also referred to as type B COPD or the “blue bloater”8,9,21) is diagnosed symptomatically by hypersecretion of bronchial mucus and a chronic or recurrent productive cough of more than 3 months’ duration and occurring each year for 2 or more successive years in patients in whom other causes have been excluded.9 For patients with chronic bronchitis and emphysema, airway obstruction is persistent and irreversible. The National Center for Health Statistics reports a 3:1 ratio of annual cases of chronic bronchitis to emphysema.23
Pathogenesis
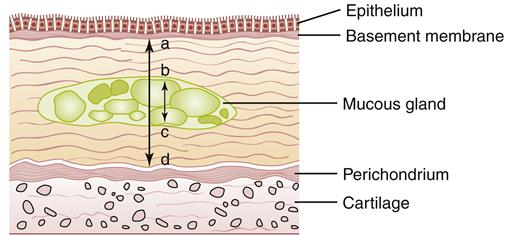
Pathologic changes in the airway include chronic inflammation and swelling of the bronchial mucosa resulting in scarring, increased fibrosis of the mucous membrane, hyperplasia of bronchial mucous glands and goblet cells, hypertrophy of bronchial glands and goblet cells, and increased bronchial wall thickness, which potentiates obstruction to airflow. Inflammation appears to predominantly be the result of neutrophil activity.21 Interleukin-8 levels are elevated, indicating sustained attraction of neutrophils to the site of inflammation. CD8 T-lymphocyte levels are also elevated. During acute exacerbations, bronchial biopsy specimens have a 30-fold increase in the number of eosinophils.21 Figures 22-7 and 22-8 show the histologic changes seen in chronic bronchitis. Hypertrophy of mucosal glands and goblet cells leads to increased mucus production; the mucus then combines with purulent exudate to form bronchial plugs. Chronic bronchitis patients often display bacterial colonization with H. influenzae and S. pneumoniae.21 The mucociliary clearance action is impaired or lost, and some areas of ciliated columnar epithelium are replaced by squamous cells.21 Ciliary dysfunction occurs because of a decreased number of cilia and decreased action of available cilia.
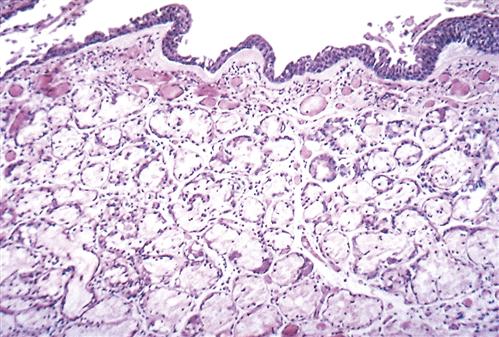
Note slight desquamation of mucosal epithelial cells and marked thickening (approximately twice the normal thickness) of mucous gland layer. Vascular congestion is evident. (From Kumar V et al: Robbins basic pathology, ed 9, Philadelphia, 2013, Saunders.)
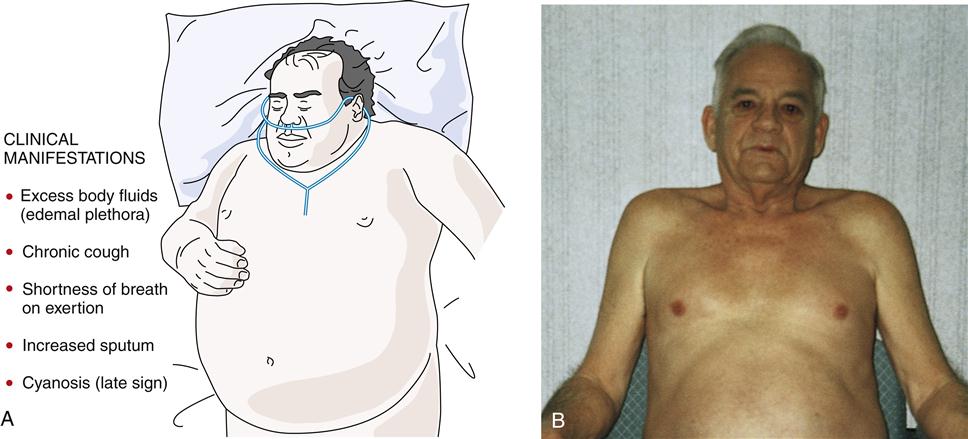
Often the inflammatory and fibrotic changes extend into the surrounding alveoli. The narrowed airways and the mucous plugs prevent proper oxygenation and potentiate airway obstruction. High airflow resistance increases the work of breathing, leading to increased oxygen demands. In areas of greater obstruction to airflow, alveoli empty and fill more slowly, leading to ventilation-perfusion (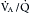 ) mismatch, thus lowering arterial oxygenation. The chronic bronchitis patient may appear as the “blue bloater” (Figure 22-9), characterizing the pathophysiologic process of oxygen desaturation (cyanosis) and edema associated with right-sided heart failure in advanced disease or exacerbations.
) mismatch, thus lowering arterial oxygenation. The chronic bronchitis patient may appear as the “blue bloater” (Figure 22-9), characterizing the pathophysiologic process of oxygen desaturation (cyanosis) and edema associated with right-sided heart failure in advanced disease or exacerbations.
The involvement of small pulmonary arteries related to inflammation in the bronchial walls and the compensatory vasoconstriction of pulmonary blood vessels from hypoxia produce pulmonary hypertension. In addition, widespread bronchial narrowing and mucous plugging produce ventilation-perfusion mismatch with hypoxemia and hypercarbia from impeded ventilation. The combination of hypoxia and hypercarbia increases pulmonary artery resistance and pulmonary hypertension.21 While the process of pulmonary hypertension continues, right ventricular end-diastolic pressures increase, leading to right ventricular dilation (cor pulmonale) and right-sided heart failure. An enlarged right heart results in increased venous pressure, liver engorgement, and dependent edema. Manifestations of heart failure may occur during exacerbations of bronchitis and subside with appropriate treatment.9,21
Destruction of bronchial walls results in dilation of airway sacs. This is termed bronchiectasis. Causes of bronchial wall destruction include infection from severe streptococcal or staphylococcal pneumonia, repeated bouts of acute bronchitis, infection with the mold Aspergillus fumigatus, presence of mucous plugs or foreign bodies, or deficiencies in immunologic response. (Refer to the Bronchiectasis section later in this chapter for a more detailed description of this disease process.) The dilated sacs contain pools of infected secretion that do not clear themselves and serve as sources of further infection that can spread to adjacent lung fields by the lymphatics or venous drainage to other areas of the body, commonly the brain. If bronchiectatic lesions are localized, surgical resection of the affected portions of lung may be helpful.
Clinical manifestations
The typical patient is an overweight man or woman (1:2 male to female ratio) in his or her thirties or forties9,21,22,24 (or older) who presents with shortness of breath on exertion, excessive amounts of sputum, chronic cough, evidence of excess body fluids (edema, hypervolemia), and a history of smoking. In addition, the patient often complains of chills, malaise, muscle aches, fatigue, loss of libido, and insomnia.
Sputum production may be variable and worsens with respiratory tract infection. Cough and sputum production are most severe in the mornings. Gradually, patients develop progressive shortness of breath on exertion. Most patients do not seek help until dyspnea becomes troublesome. By the time dyspnea on exertion is present, the disease is well advanced.21,22
In the end-stage disease process, the patient presents with signs of right-sided heart failure (distended neck veins, right ventricular heave, right ventricular gallop, and peripheral edema).21 Hypoxia leads to pulmonary hypertension. Cyanosis is a late sign.
Diagnosis
Measures used to confirm the diagnosis include chest radiography, which may show increased bronchial vascular markings, congested lung fields, an enlarged horizontal cardiac silhouette, and evidence of previous pulmonary infection. Pulmonary function tests show normal total lung capacity (TLC), increased residual volume (RV), and decreased FEV1. Early pulmonary function testing before the onset of symptoms shows increased closing volume and a decrease in the maximal midexpiratory flow rate.21 Arterial blood gas (ABG) evaluation may show elevated Paco2 and decreased Pao2 (often below 65 mm Hg); abnormal ABGs develop early in the disease process. The electrocardiogram may reveal atrial dysrhythmias and evidence of right ventricular hypertrophy. Secondary polycythemia (increased numbers of red blood cells) related to continuous or nocturnal hypoxemia is common.9,21 Hypoxemia leads to a compensatory production of red blood cells in an attempt to carry more oxygen to the body tissues.
Depending on the severity of the disease, the physical examination may reveal scattered crackles, rhonchi, and wheezes; use of accessory muscles to breathe; jugular vein distention; clubbing; and pedal and ankle edema. Table 22-1 lists the distinguishing features of both emphysema and chronic bronchitis.
TABLE 22-1
COMMON DISTINGUISHING FEATURES OF EMPHYSEMA AND CHRONIC BRONCHITIS∗
| PATIENT DATA | EMPHYSEMA (copd TYPE A) | BRONCHITIS (copd TYPE B) |
| History | ||
| Lifestyle | Smoker | Smoker |
| Weight | Weight loss | Overweight |
| Onset of symptoms | Usually after age 50 years | Usually after age 40 years |
| Sputum | Mild, mucoid | Excessive, purulent |
| Cough | Minimal or absent | Chronic; more severe in mornings |
| Dyspnea | Progressive exertional dyspnea | Mild to moderate, but may gradually progress to severe exertional dyspnea |
| Patient complaints | Dyspnea on exertion, fatigue, insomnia | Chronic cough with mucopurulent sputum, chills, malaise, muscle aches, fatigue, insomnia, loss of libido |
| Physical Signs | ||
| Edema | Absent | Present |
| Central cyanosis | Absent | Present in advanced disease |
| Use of accessory muscles to breathe | Present | Absent until end stage |
| Body build | Thin, wasted | Stocky, overweight |
| Anteroposterior chest diameter | “Barrel chest,” 1:1 ratio anteroposterior chest diameter | Normal |
| Auscultation of chest | Decreased breath sounds, decreased heart sounds, prolonged expiration | Wheezes, crackles, rhonchi, depending on severity of disease |
| Percussion | Hyperresonance | Normal |
| Jugular vein distention | Absent | Present |
| Other | Pursed-lip breathing | Evidence of right-sided heart failure (cor pulmonale) |
| General Diagnostic Tests | ||
| Chest radiography | Narrowed mediastinum; normal or small vertical heart; hyperinflation; low, flat diaphragm; presence of blebs or bullae | Congested lung fields, increased bronchial vascular markings, enlarged horizontal heart |
| Arterial blood gas analysis | Decreased Pao2 (60-80 mm Hg); increased Paco2 with advancing disease | Decreased Pao2 (<65 mm Hg); increased Paco2 |
| Electrocardiography | Normal or tall symmetric P waves; tachycardia, if hypoxic | Right axis deviation, right ventricular hypertrophy, atrial dysrhythmias |
| Hematocrit | Normal | Polycythemia |
| Pulmonary Function Tests | ||
| Functional residual capacity | Increased | Normal or slight increase |
| Residual volume | Increased | Increased |
| Total lung capacity | Increased | Normal |
| Forced expiratory volume | Decreased | Decreased |
| Vital capacity | Decreased | Normal or slight decrease |
| Static lung compliance | Increased | Normal |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree