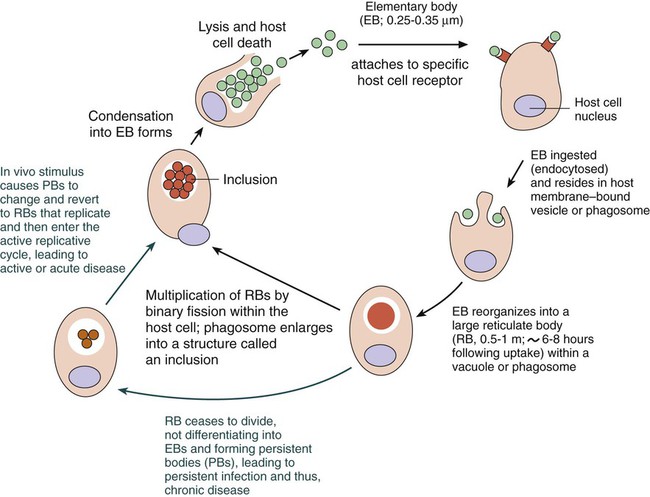
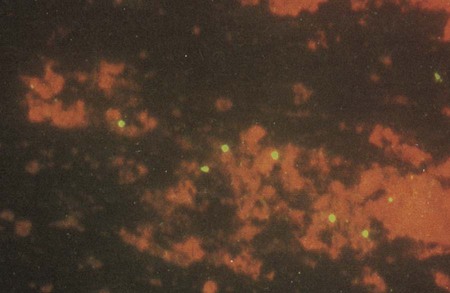
1. Define the following: bubo, proctitis, bartholinitis, salpingitis, elementary body, reticulate body, Whipple’s disease, morulae, and Donovan body. 2. Describe the general characteristics for the organisms included in this chapter including gram stain characteristics, cultivation methods (media and growth conditions), transmission and clinical significance. 3. Explain the mechanism and location for the replication of Chlamydia spp. 4. Compare the clinical manifestations and diagnosis of trachoma and other oculogenital infections associated with Chlamydia spp. 5. List the appropriate specimens used for the isolation of the organisms included in this chapter. 6. Describe the correct collection method for a specimen to be submitted for C. trachomatis screening from the female genital tract. 7. Explain the three stages associated with lymphogranuloma venereum, and compare the disease with other genital infections. 8. Describe the laboratory methods used for the diagnosis of Chlamydia infections, including sensitivity, limitations and appropriate use for culture, cytology, antigen (DFA), and nucleic acid testing (NAAT). 9. Compare hybridization and amplification nucleic acid–based testing for chlamydia. 10. Describe the triad of symptoms associated with Rickettsia spp. 11. Compare human monocytic ehrlichiosis (HME) and granulocytic anaplasmosis (HGA). 12. Distinguish and describe the three groups of Rickettsia based on mode of transmission, clinical manifestations, and intracellular growth characteristics. 13. Describe the Weil-Felix reaction, including chemical principle and limitations. 14. Describe the clinical significance for Coxiella burnetii phase I and phase II forms, including laboratory diagnosis. 15. Explain the limitations of the laboratory tests used to diagnose disease caused by the obligate intracellular and nonculturable bacteria. 16. Correlate signs, symptoms, and laboratory data for the identification of the organisms included in this chapter. The organisms addressed in this chapter are obligate intracellular bacteria or are considered either extremely difficult to culture or unable to be cultured. Organisms of the genera Chlamydia, Rickettsia, Orientia, Anaplasma, and Ehrlichia are prokaryotes that differ from most other bacteria with respect to their very small size and obligate intracellular parasitism. Three other organisms, Coxiella, Calymmatobacterium granulomatis, and Tropheryma whipplei, are discussed in this chapter because they are also difficult to cultivate or are noncultivable. Chlamydiae have a unique developmental life cycle reminiscent of parasites, with an intracellular, replicative form, the reticulate body (RB), and an extracellular, metabolically inert, infective form, the elementary body (EB). The EB cannot survive outside of a host cell for an extended period. Following infection of a host cell, the EB differentiates into a RB. The RB divides by binary fission within vacuoles. As the numbers of RB increase, the vacuole expands forming an intracytoplasmic inclusion. The RB then revert to EB, and 48 to 72 hours postinfection, the EB are released from the host cell (Figure 44-1). In addition to the replicative cycle associated with acute chlamydial infections, there is evidence that Chlamydia can persist in an aberrant form in vitro depending on the amount of interferon-gamma (IFN-γ) and tryptophan in the host cell as well as the function of the tryptophan synthase encoded by the organism. Removal of the IFN-γ or increase in tryptophan will result in the differentiation of the chlamydiae into an active EB infection. The therapeutic implications of this persistence in vivo has not yet been completely defined; however, evidence suggests that the activity of the tryptophan synthase gene in C. trachomatis differs between isolates recovered from the eye versus the genital tract. C. trachomatis, C. pneumoniae, and C. psittaci are important causes of human infection; C. psittaci and C. pecorum are common pathogens among animals. The three species that infect humans differ with respect to their antigens, host cell preference, antibiotic susceptibility, EB morphology, and inclusion morphology (Table 44-1). TABLE 44-1 Differential Characteristics among Chlamydiae That Cause Human Disease C. trachomatis infects humans almost exclusively and is responsible for various clinical syndromes. Based on MOMP antigenic differences, C. trachomatis is divided into 18 different serovars that are associated with different primary clinical syndromes (Table 44-2). TABLE 44-2 Primary Syndromes Caused by C. trachomatis C. trachomatis causes significant infection and disease worldwide. In the United States, C. trachomatis is the most common sexually transmitted bacterial pathogen and a major cause of pelvic inflammatory disease (PID), ectopic pregnancy, and infertility (see Chapter 74 for more information on PID). An estimated 3 million cases of C. trachomatis infection occur annually in the United States. In 2010, more than 1.3 million cases of C. trachomatis infection were reported to the Centers for Disease Control and Prevention (CDC), corresponding to a rate of infection of 426 per 100,000 population and a 5.1% increase over the cases reported in 2009. In fact, of all the organisms causing sexually transmitted disease reported to the CDC, only C. trachomatis cases have increased every year. Genital tract infections caused by C. trachomatis were identified most frequently in women between the ages of 15 and 24 years. It is important to note, however, that data reported to the CDC, especially with regards to Chlamydia, come as a result of screening programs that primarily target women between the ages of 15 and 24 years. C. trachomatis infections are primarily transmitted from human to human by direct contact with infected secretions. Some infections, such as neonatal pneumonia or inclusion conjunctivitis, are transmitted from mother to infant during birth. The various routes of transmission for C. trachomatis infection are summarized in Table 44-2. Although its specificity approaches 100%, the sensitivity of culture has been estimated at between 70% and 90% in experienced laboratories. Limitations of Chlamydia culture contributing to the lack of sensitivity include prerequisites to maintain viability of patient specimens by either rapid or frozen transport and to ensure the quality of the specimen submitted for testing (i.e., endocervical specimens devoid of mucus and containing endocervical epithelial or metaplastic cells or urethral epithelial cells). In addition, successful culture requires a sensitive cell culture system and a minimum of at least 2 days turnaround time between specimen receipt and the availability of results. Despite these limitations, culture is still recommended as the test of choice in some situations (Table 44-3). As of this writing, only chlamydia cultures should be used in situations with legal implications (e.g., sexual abuse) when the possibility of a false-positive test is unacceptable. Local and state requirements may vary. TABLE 44-3 Use of Different Laboratory Tests to Diagnose C. trachomatis Infections DFA, direct fluorescent antibody staining; EIA, enzyme immunoassay; NAH, nucleic acid hybridization; NAAT, nucleic acid amplification test. *Must be confirmed in a population with a low prevalence (<5%) of C. trachomatis infection. †EIA can be used on urine from symptomatic men but not on urine from older men. Also, a positive result must be confirmed in a population with a low prevalence of C. trachomatis infection. Modified from Centers for Disease Control and Prevention: Recommendations for the prevention and management of Chlamydia trachomatis infections, MMWR 42(RR-12):1-39, 1993; Centers for Disease Control and Prevention: Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections, MMWR 51(RR-15):1-27, 2002; Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines, 2010, MMWR 59(RR-12):1, 2010. Direct fluorescent antibody (DFA) staining methods use fluorescein-isothiocyanate conjugated monoclonal antibodies to either MOMP or LPS of C. trachomatis to detect elementary bodies in smears of clinical material (Figure 44-2). The sensitivity and specificity of DFA are similar to those of culture. Chlamydial antigen can also be detected by enzyme immunoassays (EIA). Numerous U.S. Food and Drug Administration (FDA)-approved kits are commercially available. These assays use polyclonal or monoclonal antibodies that detect chlamydial LPS. These tests are not species-specific for C. trachomatis and may cross-react with LPS of other bacterial species present in the vagina or urinary tract and thereby produce a false-positive result. • Performing a second nonculture test that identifies a C. trachomatis antigen or nucleic acid sequence that is different from that used in the screening test • Using a blocking antibody or competitive probe that verifies a positive test result by preventing attachment of a labeled antibody or probe used in the standard assay
Obligate Intracellular and Nonculturable Bacterial Agents
Chlamydia
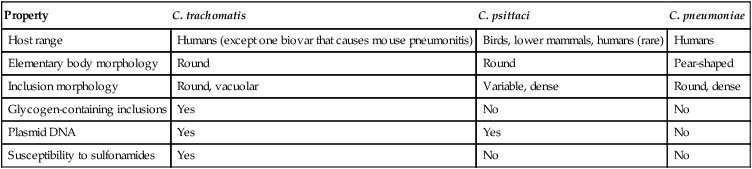
Property
C. trachomatis
C. psittaci
C. pneumoniae
Host range
Humans (except one biovar that causes mouse pneumonitis)
Birds, lower mammals, humans (rare)
Humans
Elementary body morphology
Round
Round
Pear-shaped
Inclusion morphology
Round, vacuolar
Variable, dense
Round, dense
Glycogen-containing inclusions
Yes
No
No
Plasmid DNA
Yes
Yes
No
Susceptibility to sulfonamides
Yes
No
No
Chlamydia Trachomatis
General Characteristics
Serovars
Clinical Syndrome
Route(s) of Transmission
A, B, Ba, C
Endemic trachoma (multiple or persistent infections that ultimately lead to blindness)
Hand to eye from fomites, flies
L1, L2, L2a, L3
Lymphogranuloma venereum
Sexual
D-K
Urethritis, cervicitis, pelvic inflammatory disease, epididymitis, infant pneumonia, and conjunctivitis (does not lead to blindness)
Sexual, hand to eye by autoinoculation of genital secretions; eye to eye by infected secretions; neonatal
Epidemiology and Pathogenesis
Laboratory Diagnosis
Cultivation.
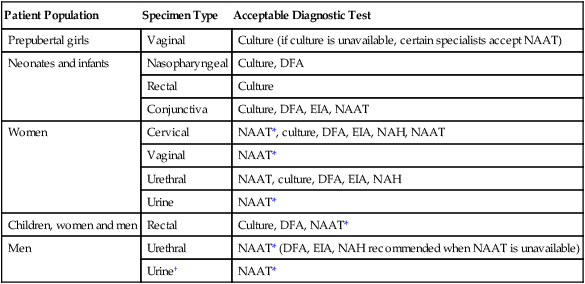
Patient Population
Specimen Type
Acceptable Diagnostic Test
Prepubertal girls
Vaginal
Culture (if culture is unavailable, certain specialists accept NAAT)
Neonates and infants
Nasopharyngeal
Culture, DFA
Rectal
Culture
Conjunctiva
Culture, DFA, EIA, NAAT
Women
Cervical
NAAT*, culture, DFA, EIA, NAH, NAAT
Vaginal
NAAT*
Urethral
NAAT, culture, DFA, EIA, NAH
Urine
NAAT*
Children, women and men
Rectal
Culture, DFA, NAAT*
Men
Urethral
NAAT* (DFA, EIA, NAH recommended when NAAT is unavailable)
Urine†
NAAT*
Direct Detection Methods
Antigen Detection and Nucleic Acid Hybridization.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree