Chapter Twenty-Three. Nutrition and metabolism during pregnancy
Nutrition
Nutrition is based on two fundamental areas of science: biochemistry and physiology. For practical purposes, this section will focus on a brief overview of basic nutrition physiology: general food groups, energy and nutrients and metabolism. Nutrients are utilised by the body for tissue growth, maintenance and repair. They can be divided into six categories: three major nutrients (macronutrients) are carbohydrates, lipids and proteins; and micronutrients including vitamins and minerals which are required in small amounts as well as water which is considered as a food because of its role as a solvent. Most foods provide a combination of nutrients, and water makes up 60% of the volume of food intake.
Food groups
There are four food groups which must be eaten in order to provide a balanced diet. The types and a general guide on the daily food choices are:
1. Grains, bread, cereal, rice and pasta 6–11 servings.
2. Fruits 2–4 servings, vegetables 3–5 servings.
3. Meat, poultry and fish 2–3 servings.
4. Dairy products, milk, cheese and yoghurt 2–3 servings.
Tiran (1997) adds a fifth group—fats and sugary foods. The five groups can be arranged in a pyramid, showing the quantities in relation to the health benefits of consumption (Fig. 23.1).
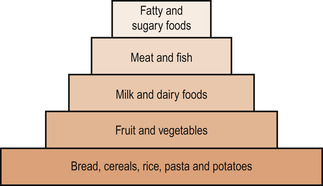 |
| Figure 23.1 The ‘good food guide’, showing the five main groups of food. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
Carbohydrates
Carbohydrate is the main source of energy in our food although the other macronutrients can also be metabolised to yield energy. According to their structure, there are three types of carbohydrates; their main food sources are explained below.
1. Monosaccharides (single sugars):
a. Glucose, which is the ultimate example (corn syrup, processed foods).
b. Fructose (fruits, honey).
c. Galactose (milk).
2. Disaccharides (double sugars):
a. Sucrose, which is made from glucose and fructose (sugar).
b. Lactose, which is made from glucose and galactose (milk).
c. Maltose, which is made from two glucoses (commercial malt product of starch breakdown, intermediate sweetener in food products).
3. Polysaccharides (multiple sugars or complex carbohydrates):
a. Starch (grains, legumes, root vegetables).
b. Glycogen (liver and muscle meats).
c. Dietary fibre (whole grain, fruit and vegetables, seeds and nuts).
Carbohydrates play a fundamental role in the physiology of the body. Glucose, for example, is the ultimate common refined body fuel that is oxidised in cells to give energy; it is important to maintain a certain range in the blood of between 3.9 and 7.8 mmol/L to allow normal functioning. Cellulose is a form of plant carbohydrate (dietary fibre) that the human digestive system cannot process. It provides roughage to increase the bulk of faeces, thus facilitating defecation. Dietary fibres are also believed to have a role in the management of serum lipid and glucose levels; they are therefore helpful in prevention and management of chronic diseases such as diabetes and cardiovascular disease.
Carbohydrate metabolism
The Krebs (tricarboxylic acid or citric acid) cycle
When glucose in the circulating blood arrives at the tissues it is taken up by the cells by facilitated diffusion under the influence of insulin. Glucose is taken to the cells’ mitochondria where it is oxidised to form energy via the Krebs cycle (Figure 23.2 and Figure 23.3). A series of reactions are involved when glucose, a 6-carbon molecule, is broken down into two 3-carbon molecules of pyruvic acid in a process called glycolysis.
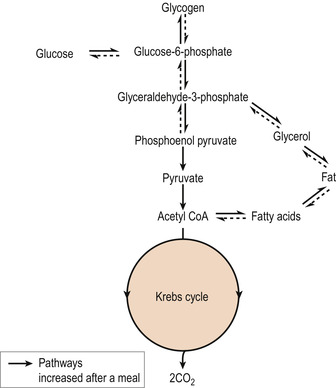 |
| Figure 23.2 A summary of glucose metabolism after a meal. (From Hinchliff S M, Montague S E 1990, with permission.) |
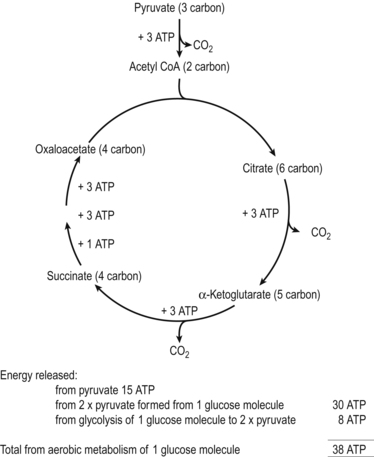 |
| Figure 23.3 A summary of the Krebs cycle. (From Hinchliff S M, Montague S E 1990, with permission.) |
Pyruvic acid is broken down further to a 2-carbon molecule called acetic acid and the released carbon atom forms carbon dioxide by combining with oxygen. The acetic acid now combines with coenzyme A (a derivative of a B complex vitamin, pantothenic acid) to form the enzyme acetyl coenzyme A (acetyl CoA). Acetyl CoA enters the Krebs cycle to undergo changes mediated by enzymes; ATP, water and carbon dioxide are formed in the process of oxidation.
The Krebs cycle consists of a series of eight separate biochemical reactions. This cycle of reactions is like one revolution of a wheel with acetyl CoA sitting at the top. Each glucose molecule produces two pyruvic acid molecules and allows two turns of the cycle. Carbon dioxide produced when the excess carbon atoms unite with oxygen and surplus hydrogen atoms need to be disposed of. Two hydrogen carrier molecules, nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD), perform this function. The transfer of the hydrogen atoms converts the compounds into NADH and FADH 2, respectively.
ATP synthesis
Energy is stored in carbon–hydrogen bonds in food but cells cannot use energy in this form. They must convert it into the high-energy phosphate bonds of adenosine triphosphate (ATP) (Guyton & Hall 2006). ATP consists of adenosine with three phosphate groups attached. When a bond between adenosine (a nucleotide) and one of the phosphate groups is split, large amounts of energy are released and adenosine diphosphate plus an inorganic phosphate molecule (P i) is formed. Some of the energy produced is in the form of heat and helps to maintain the temperature of the body. This can be written as:
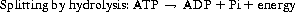
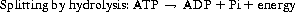
Cellular respiration
Aerobic cellular respiration
The main role of the Krebs cycle is to prepare the hydrogen acceptors for entry into the electron transport chain (respiratory chain) in the inner mitochondrial membrane. Electron transfer molecules are mostly brightly coloured protein-bound iron-containing pigments called cytochromes. These are arranged in a specific order which allows high-energy electrons to pass through a chain of reactions, resulting in a lowering of the energy levels at each step. At the end of this chain, the electrons are passed to the final electron acceptor, which is inspired oxygen.
The stepwise release of energy during the progression through the electron transport chain is used to pump protons into the intermembrane space. This creates an electrochemical proton gradient across the mitochondrial inner membrane which temporarily stores the energy. Protons flow back across the membrane through the enzyme ATP synthetase, providing the energy to attach a phosphate group to ADP to create a further 32 ATP molecules for each glucose molecule processed. The total production of ATP for one turn of the cycle is therefore 38 molecules. NAD and FAD are released to capture more hydrogen and begin the energy transfer again. This process, which uses oxygen and phosphate, is called oxidative phosphorylation or aerobic respiration (Marieb 2008).
Anaerobic cellular respiration
If there is no oxygen available, a few molecules of ATP are synthesised during glycolysis but the process cannot proceed further. Only two molecules of ATP are available for each glucose molecule. This is called substrate-level phosphorylation (Marieb 2008). The energy remains trapped in the molecules of pyruvic acid, and lactic acid is formed (anaerobic respiration). An oxygen debt arises and metabolic acidosis is created (Guyton & Hall 2006). When oxygen is available, the lactic acid is gradually converted to pyruvic acid and fed into the Krebs cycle.
Lipids
Lipid is the chemical name for fats and fat-related compounds. The most common source of lipids is in the form of triglycerides. Triglycerides are made up of fatty acids and glycerol. Fats provide the highest-density source of energy for the body. Carbohydrates can also be converted to fat and stored in the adipose tissue. Fat is essential for maintaining health; what is harmful is excess fat intake (more than 30% of the total calories). Fatty acids, the common structural units of lipids, are also a refined fuel form which is preferred by some cells (e.g. heart muscle) over glucose.
Fatty acids consist of carbon, hydrogen and oxygen atoms. If a given fatty acid is filled with as much hydrogen as it can take, the fatty acid is saturated with hydrogen and the lipids containing them are called saturated fats. Saturated fats are mainly in animal fats such as meat and dairy products. Monounsaturated fats are fats containing fatty acids with one less hydrogen atom, creating one double bond between carbon atoms. The main sources of such fats are olive oils and canola oil, which is derived from the rape seed. Finally, lipids made mainly from unsaturated fatty acids with two or more places unfilled with hydrogen, creating double bonds, are called polyunsaturated. These are also from plant sources (Rodwell-Williams 1999).
Humans can synthesise saturated and monounsaturated fatty acids but there are some that the liver cannot make such as the n-3 and the n-6 families of long-chain polyunsaturated fatty acids (LC-PUFA). From this family, linolenic acid (LA) and α-linolenic (ALA) are essential fatty acids and must be included in the dietary intake. Arachidonic, eicosapentanoic and decosahexanoic acids (DHA) can all be synthesised from LA and ALA, but, if the supply of linoleic and α-linolenic acid is limited, they can become essential fatty acids. These fatty acids are found in vegetable oil and oily fish.
Body fat stores and functions
Body fat is mainly stored under the skin and around the abdominal organs. It is continually being interchanged with the fats circulating in the bloodstream and being metabolised for use as fuel. The dietary reference value for total fat intake is that 35% of energy should be provided by fat. Fats are essential for many of the body’s functions:
• They are a source of energy.
• They are involved in the absorption of the fat-soluble vitamins.
• Triglycerides provide the major fuel for energy for hepatocytes and skeletal muscle.
• Phospholipids are a component of myelin sheaths that surround larger nerves and all cell membranes.
• Fatty deposits act as protective cushions for the vital organs such as eyes and kidneys.
• Fats provide an insulating layer under the skin.
• Prostaglandins are formed from linoleic acid; they play a role in smooth muscle contraction and inflammatory responses.
• Fats are part of the cell membrane structure.
• Fats are involved in cell metabolism; combinations of lipids and protein, called lipoproteins, carry lipids in the blood to cells.
• They are involved in nerve impulse transmission.
Lipid metabolism
The energy obtained from fat breakdown or lipolysis is twice that obtained from the metabolism of glucose or protein (4 kcal/g each), providing 9 kcal/g. Any excess fat is stored as adipose tissue in subcutaneous tissues and retroperitoneal tissue. When fat stores are needed for energy production, as in glucose shortage, they are mobilised from the stores under the influence of growth hormones or cortisol. They are taken to the liver where the triglycerides are broken down into free fatty acids and glycerol which are released into the blood.
Glycerol is converted to one of the intermediate products of glycolysis called glyceraldehyde phosphate and is thus assimilated into the energy-releasing process. Further oxidation of the fatty acids releases the 2-carbon acetic acid which is fused to coenzyme A to form acetyl CoA, which enters the Krebs cycle. Fatty acids cannot be used for gluconeogenesis (glucose synthesis) because they enter the cycle beyond the pyruvic acid stage when the changes are irreversible.
In the presence of oxygen and glucose, fatty acids are converted to acetyl CoA, which enters the Krebs cycle. If no glucose is available (e.g. in starvation, diabetes mellitus, some slimming diets or hyperemesis gravidarum) metabolism of a large amount of fat may occur, acetyl CoA accumulates and the liver converts the molecules to ketone bodies, i.e. acetoacetic acid and β-hydroxybutyric acid, which accumulate in the blood. These can be oxidised to release energy but metabolic acidosis will occur.
Cholesterol
Despite common perception, cholesterol is not a fat itself but is a fat-related compound vital in human metabolism. Cholesterol belongs to the steroid family and helps in production of vitamin D, formation of bile acids, digestion and absorption of fats, steroid hormones and cell membranes. Cholesterol is found in all animal foods, mainly in egg yolk and organ meats such as kidneys and liver. Even with no dietary intake of cholesterol, it is synthesised in the liver.
Bile salts, steroid hormones and cell membranes are formed from cholesterol. Cholesterol is found in the blood—mainly in combination with a protein carrier—as lipoproteins, of which there are three types:
1. High-density lipoproteins (HDLs);
2. Low-density lipoproteins (LDLs);
3. Very low-density lipoproteins (VLDLs).
It is also thought that cholesterol is laid down as atheromatous plaques in arterial walls in the form of LDLs and VLDLs. A high ratio of HDLs to LDLs and VLDLs may offer protection against ischaemic heart disease (Marieb 2008). The ratio of HDLs to LDLs and VLDLs has been shown to be increased in vegetarians, people whose fat intake is largely unsaturated and who take regular exercise and reduced in smokers.
Proteins
Animals (including humans) can synthesise protein from amino acids but are unable to synthesise amino acids de novo. Plants can synthesise amino acids from carbon dioxide, water and nitrogen. Therefore, human dietary sources of amino acids/proteins are plants or other animals. Amino acids can be converted to each other through a process in the liver called transamination. Those amino acids that the body either cannot make or cannot make in sufficient amount are called essential or semi-essential amino acids. The best source of essential amino acids is animal products.
Protein foods that have all the essential amino acids such as eggs, meat and human breast milk are said to have a high biological value. Protein foods from plant sources tend to have a lower biological value and should be eaten as a mixture, for their proteins complement each other, hence improving their biological values. Strict vegetarians can obtain adequate levels of essential amino acids by varying their diet carefully. For instance, cereals and legumes contain all the essential amino acids when taken together. Examples of such meals are West Indian rice and peas or the Middle Eastern meals of dishes including a mixture of peas and beans eaten with bread.
The functions of proteins
Proteins include important structural molecules such as muscle protein, collagen and elastin in connective tissue and keratin in skin. They produce new tissue and are therefore essential for growth, recovery from injury, pregnancy and lactation. Proteins also function as hormones, enzymes and transport molecules such as haemoglobin. Amino acids can be used to synthesise proteins or can be converted to glucose to provide energy. These uses depend on adequacy of calorie intake, nitrogen balance (balance between protein synthesis and protein breakdown) and the influence of hormones (anabolic hormones such as growth hormone and the sex hormones against the glucocorticoids produced in stress which enhance protein breakdown and the conversion of amino acids to glucose).
The amount of protein needed in the diet is influenced by age, size, metabolic rate and nitrogen balance. The recommended daily intake (RDA) is 0.8 g/kg body weight in non-obese individuals. This is equivalent to 60 g of fish or meat and a glass of milk daily. Meat eaters in developed countries eat far in excess of the daily amount needed while some people in developing countries rarely eat meat.
Amino acids
At least 50 g a day is needed to maintain nitrogen balance and to provide for growth and repair of tissues. Amino acids cannot be stored by the body. However, the liver can interconvert amino acids by utilising the eight essential amino acids to synthesise the non-essential amino acids. Any excess amino acids are broken down by the liver by a process of deamination. The nitrogen portion is converted into urea, which enters the blood and is excreted by the kidney.
Proteins are needed by the body for the following functions:
• The formation of new cells.
• The manufacture of enzymes, hormones and antibodies.
• The transport molecules such as haemoglobin.
• Plasma proteins which act as buffers to maintain acid–base balance.
• The control of osmotic pressure between body fluid compartments.
• Amino acids can be used in gluconeogenesis once glucose stores are depleted.
Vitamins
Vitamins are needed in small amounts for growth and health (Marieb 2008). They mainly function as coenzymes to assist in the catalysis of chemical processes and metabolism in the body. The human body is unable to synthesise most vitamins with the exception of vitamin K and some of the B vitamins. Vitamins are distinguished usually according to their solubility in either fat or water. Some vitamins, such as vitamins A, D, E and K, are fat-soluble and are absorbed bound to digested lipids. The water-soluble vitamins, such as most of the B complex and vitamin C, are absorbed with water from the gastrointestinal tract. A normal varied diet should provide them all. Excessive intake can create as many health problems as insufficient intake. Details of the vitamins are given in Box 23.1.
 BOX 23.1
BOX 23.1 THE VITAMINS
Fat-soluble vitamins
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


