CHAPTER 14 Nuclear Structure and Dynamics
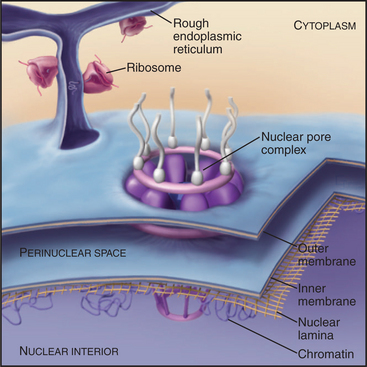
The nucleus houses the chromosomes together with the machinery for DNA replication and RNA transcription and processing (Fig. 14-1). Immature RNAs must be kept apart from the translational apparatus because eukaryotic genes are transcribed into RNAs containing noncoding intervening sequences that must be removed by splicing to assemble mature RNA molecules with a continuous open reading frame. Sequestration of immature RNAs is one function of the nuclear envelope, two concentric membrane bilayers that separate the nucleus and cytoplasm. The nuclear envelope also regulates the movement of proteins, such as transcription factors, into and out of the nucleus.

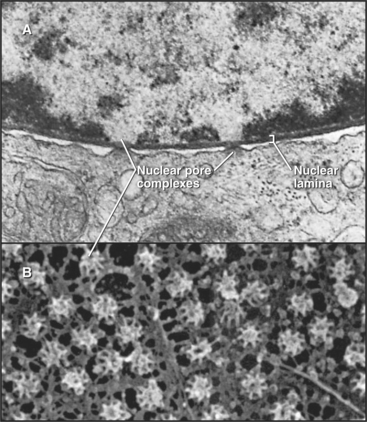
Figure 14-1 ELECTRON MICROGRAPH OF A THIN SECTION OF A NUCLEUS FROM A CANCER CELL WITH THE MAJOR FEATURES LABELED.
(Courtesy of Scott Kaufmann, Mayo Clinic, Rochester, Minnesota.)
This chapter describes what is known about the structure of the nucleus, the nuclear envelope, and the transport of macromolecules into and out of the nucleus. Much is known about the mechanisms of DNA replication (see Chapter 42), RNA transcription and processing (see Chapters 15 and 16), and nuclear trafficking of macromolecules. Currently, less is known about the structural organization of the nucleus and the role of its specialized subcompartments in these nuclear functions.
Overall Organization of the Nucleus
Studies in which entire individual chromosomes are labeled by in situ hybridization (chromosome painting; see Fig. 13-11) reveal that chromosomes tend to occupy discrete regions within the nucleus called chromosome territories. The boundaries of these territories are not absolute, as in some cases an active gene is located well outside its respective territory (see Chapter 13). The poorly defined region between adjacent territories is referred to as the interchromosomal domain. Most RNA transcription, processing, and transport are thought to occur either within or at the boundary of this domain. Although the nucleoplasm is very crowded with chromosomes and RNPs, many nuclear proteins can diffuse rapidly through the nucleus, possibly by moving in the interchromosomal domain.
Specialized Subdomains of the Nucleus
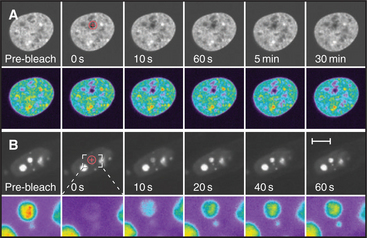
Cell nuclei contain numerous discrete subdomains or bodies with distinctive structural organizations and/or biochemical composition (Fig. 14-2 and Table 14-1). The most prominent of these is the nucleolus, discussed in the next section. Although these subdomains are often referred to as organelles, unlike cytoplasmic organelles, nuclear subdomains are not membrane bounded. In fact, many proteins that have been examined by the fluorescence recovery after photobleaching technique (see Fig. 6-3) exhibit a relatively rapid exchange between a respective body and a nucleoplasmic pool of the protein. Therefore, although these bodies are discrete at steady state, they represent highly dynamic associations of macromolecular complexes. These domains, like the nucleolus, reflect the functional compartmentalization of the nucleoplasm.
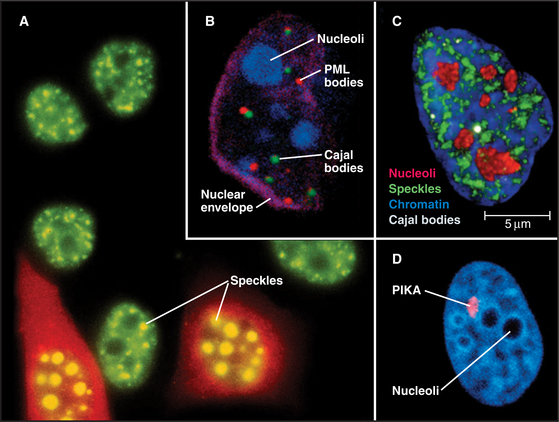
Figure 14-2 examples of major subnuclear structures. A, Components involved in RNA processing are scattered throughout the nucleus but concentrated in domains called speckles that are rich in interchromatin granules. Inhibition of RNA processing causes splicing components to accumulate in enormous concentrations of interchromatin granule clusters. Several cells were injected with a short oligonucleotide that disrupts the function of the U1 snRNP in RNA processing (see Chapter 16), and were then stained with an antibody recognizing the Sm splicing components (green). The injected cells were marked by introducing an inert fluorescent dextran marker into the cytoplasm (red). B, Nucleus with simultaneous staining of nucleoli (blue), PML nuclear bodies (red), Cajal bodies (green), and the nuclear envelope (purple). C, Nucleus with simultaneous staining of chromatin (blue), nucleoli (red), speckles (green), and Cajal bodies (white). D, Nucleus with simultaneous staining of DNA (blue) and the polymorphic interphase karyosomal association (PIKA)/OPT domain (red). Nucleoli appear as unstained areas. A number of proteins involved in the sensing and repair of DNA damage concentrate in the PIKA.
(A, Courtesy of David Spector, Cold Spring Harbor Lab, New York. B–C, Courtesy of Angus Lamond, University of Dundee, Scotland. D, Courtesy of William S. Saunders and William C. Earnshaw.)
Table 14-1 MAJOR NUCLEAR SUBDOMAINS
| Structure | Comments |
|---|---|
| Cajal bodies | Formerly known as coiled bodies. About 0.2 to 1 μm in diameter, Cajal bodies have a coiled fibrous substructure. First identified by electron microscopy, up to 10 of these structures are seen per cell. They contain a characteristic protein called p80-coilin. They may be involved in snRNP and snoRNP assembly. |
| GEMs | GEMs are usually found paired with Cajal bodies, which they may overlap. They contain the survival of motor neurons (SMN) protein, which is encoded by the gene mutated in spinal muscular atrophy, a severe, inherited, human, muscular wasting disease. SMN and its cofactors appear to play an essential role in the assembly and maturation of snRNPs (see Chapter 16). |
| Nuclear bodies | Function unknown. 5 to 20 spots within the nucleus. Originally observed in electron micrographs of cells following hormonal treatments. However it is not clear that all nuclear bodies described in various cell types are structurally or functionally homologous. A marker antigen for some types of nuclear bodies (called PBC 95K—Mr 95 kD) is recognized by autoantibodies from patients with primary biliary cirrhosis. Some may correspond to PML bodies. |
| Nucleolus | The nucleolus (typically 1 to 5 structures of 0.5 to 5 μm diameter in mammalian cell nuclei) is the site of rRNA transcription and processing, as well as of preribosomal assembly. It is also the site of processing of several other noncoding RNAs, including the RNA component of the signal recognition particle (SRP—Chapter 20). |
| PIKA | The polymorphic interphase karyosomal association (PIKA) was later rediscovered and termed the OPT domain. The PIKA may be up to 5 μm in diameter during G1 phase, but its morphology and number vary across the cell cycle. It appears to correspond to sites of sensing or repair of DNA damage as well as concentrations of certain transcription factors. |
| PML bodies | Also known as PODs and ND10, 10 to 30 of these structures are scattered throughout the nucleus. They are have links with human disease, and in some cases appear to be targeted during viral infections. Fusion of the marker protein PML to the α-retinoic acid receptor is often found in acute promyelocytic leukemia (hence the name PML), in which the PML bodies appear highly fragmented. The link with cancer appears significant, as treatments that are effective against PML appear to restore the normal morphology of PML bodies (see text). |
| Speckles | Speckles are concentrations of components involved in RNA processing. They often correspond to clusters of interchromatin granules seen by electron microscopy. They may serve as storage depots of splicing factors, or they may play a more active role in splicing factor modification and/or assembly. |
When factors involved in RNA processing are detected by fluorescence microscopy, 20 to 50 bright speckles are seen against a diffuse background of nucleoplasmic staining (Fig. 14-2). The diffuse staining probably corresponds to splicing factors associated with perichromatin fibrils at the thousands of sites where RNA transcription and processing take place. Speckles are less prominent in cells that transcribe RNA at high levels, and they become strikingly prominent when RNA processing is inhibited (Fig. 14-2).
When cells enter mitosis, speckles disperse as RNA-processing factors redistribute diffusely throughout the cytoplasm. During telophase, processing factors reaggregate in the cytoplasm into punctate structures termed mitotic interchromatin granule clusters. These factors are subsequently imported into the nucleus at the completion of mitosis.
Cajal bodies (formerly known as coiled bodies) are compact structures about 0.3 to 1.0μm in diameter (Fig. 14-2) that resemble balls of tangled threads in the electron microscope. Nuclei contain 1 to 10 Cajal bodies, which accumulate many factors involved in mRNA processing, as well as a number of nucleolar components, but lack non-snRNP protein-splicing factors that are present in speckles. They also contain an 80-kD human autoantigen of unknown function, called p80-coilin. In contrast to speckles, Cajal bodies disperse when transcription and splicing are blocked, and they are particularly prominent in rapidly growing cells with high levels of gene expression. However, like speckles, some Cajal bodies disassemble during mitosis and reform during the G1 phase after transcription is reinitiated. Cajal bodies are connected with the maturation of newly imported snRNP and snoRNP particles (see Chapter 16). They may also have other functions, including possibly regulating expression of snRNA gene clusters.
Mammalian nuclei also contain about 10 to 30 bodies, varying in size from 0.3 to 1μm, known as promyelocytic leukemia (PML) bodies (other names are listed in Table 14-1) that are often juxtaposed with Cajal bodies (Fig. 14-2). PML bodies were initially defined by the presence of a protein called PML, and other components have since been identified. PML has a RING finger amino acid sequence motif and is therefore likely to be a ligase for ubiquitin or ubiquitin-like proteins (see Fig. 23-8). Its targets are unknown, but several components of PML bodies are conjugated to the ubiquitin-like protein SUMO-1.
The Nucleolus: The Most Prominent Nuclear Subdomain
The nucleolus, first described only five years after the nucleus, in 1835, is the most conspicuous and best-characterized nuclear subdomain (Fig. 14-3). Most mammalian cell nuclei have one to five nucleoli, which are specialized regions 0.5 to 5.0μm in diameter surrounding transcriptionally active ribosomal RNA (rRNA) gene clusters. Within nucleoli occur the bulk of the steps for ribosome biogenesis, from the transcription and processing of rRNA to the initial assembly of ribosomal subunits. The ribosome is a complex macromolecular machine with four different structural RNA molecules and about 85 proteins that are assembled into two subunits (see Figs. 17-6 and 17-7). rRNA transcription by RNA polymerase I comprises nearly one half of total cellular RNA synthesis in some cell types. This high level of synthesis is necessary to produce about 5 million ribosomes in each cell cycle, more than 30 every second in budding yeast.
Over 690 proteins associate stably with human nucleoli. Many more may associate transiently, and this composition changes to reflect different metabolic states of the cell (Fig. 14-4). Many of these nucleolar proteins are involved with either ribosomal RNA synthesis and modification or with ribosome subunit assembly. Surprisingly, the functions of many (˜80) other nucleolar proteins remain unknown and may reflect the involvement of nucleoli in other biological processes. Other stable RNAs, including the RNA component of the signal recognition particle (SRP; see Fig. 20-5), are also processed in the nucleolus, and the nucleolus might have other as yet undiscovered functions.
Ribosomal Biogenesis in Functionally Distinct Regions of the Nucleolus
The nucleolus contains three morphologically distinct regions in thin sections viewed by transmission electron microscopy (Fig. 14-3). Fibrillar centers contain concentrations of rRNA genes, together with significant amounts of RNA polymerase I and its associated transcription factors. Actively transcribed ribosomal genes are found near the border between the fibrillar centers and a dense fibrillar component that surrounds them. The granular component is the site for many steps in ribosome subunit assembly and is made up of densely packed clusters of preribosomal particles 15 to 20 nm in diameter.
Ribosomal RNA loci have a modular organization, with genes alternating with spacer regions in large tandemly arranged clusters (see Fig. 16-9). The repeat unit in this array (gene plus spacer) is approximately 40,000 base pairs in humans. Humans have approximately 300 to 400 copies of the ribosomal DNA (rDNA) repeat unit located in clusters on chromosomes 13, 14, 15, 21, and 22. Usually, only a fraction of these genes is actively transcribed. An additional rRNA, 5S, is encoded by distinct genes and transcribed by RNA polymerase III (see Fig. 15-10).
A simple yet efficient mechanism guarantees a balance between the RNA components of the two ribosomal subunits. The major rRNA components are encoded by a single precursor RNA molecule. In humans, this 13,000-base precursor is commonly described by its sedimentation coefficient in sucrose gradients as 45S. Following its transcription, the RNA precursor is processed in a series of cleavages to yield the 18S, 5.8S, and 28S rRNA molecules (see Fig. 16-9). In addition to the cleavages, rRNA processing also involves extensive base and sugar modifications, including approximately 100 2′-O-methyl ribose and approximately 90 pseudouridine residues per molecule. The earliest stages of rRNA processing probably occur in the dense fibrillar component of the nucleolus. Later stages take place in the granular component. Ribosomal protein synthesis occurs in the cytoplasm on free ribosomes, and the newly synthesized proteins are transported into the nucleus for assembly into ribosomes, predominantly in the granular component.
Disassembly of the Nucleolus during Mitosis
The nucleolus disassembles during each mitotic cycle, starting with the dispersal of the dense fibrillar and granular components during prophase. This disassembly is apparently driven by specific phosphorylation of nucleolar proteins. Ultimately, the fibrillar centers alone remain associated with the mitotic chromosomes, forming what are termed nucleolus-organizing regions (NORs [Fig. 14-3B]). NORs are often the sites of a prominent secondary constriction of the chromosome. (The primary constriction is the centromere.) The nucleolar proteins nucleolin and RNA polymerase I remain bound at NORs as cells enter and exit mitosis.
Nucleolar reformation begins in telophase as processing factors and unprocessed pre-RNA remaining from the previous cell cycle associate with NORs (10 in human), which then cluster into one to five foci. Next, a wide variety of nucleolar components assemble into particles termed prenucleolar bodies that associate with the NORs in a process requiring transcription of the rRNA genes. Normally, nascent transcripts, rather than ribosomal genes, nucleate assembly of the nucleolus in each cell cycle. If antibodies to RNA polymerase I are microinjected into mitotic cells, rRNA transcription is blocked, and nucleoli do not reform in the next G1 phase.
Structure of the Nuclear Envelope
The nuclear envelope provides a selective permeability barrier between the nuclear compartment and the cytoplasm (Fig. 14-5). This barrier ensures that only fully processed mRNAs are delivered to ribosomes for translation into protein. In addition, various chromosomal events, including DNA replication and expression of certain genes, are regulated, at least in part, by changes in the ability of factors to move from the cytoplasm into the nucleus.
Structure and Assembly of the Nuclear Lamina
The nuclear lamina is a protein meshwork, typically 20 to 40 nm thick, composed of type V intermediate filament proteins called nuclear lamins (Fig. 14-6). Mammalian lamins are generally divided into two families. Lamin A is encoded by a gene that gives rise to four polypeptides (including lamin C) by alternative splicing (see Fig. 16-6). Members of the lamin B family are the products of two distinct genes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree