Active substance
Physical form
Indication/therapeutic class
Preparation
Azelastine HCl
Solution
Allergic rhinitis/antihistamine
Allergodil®, Allergocrom®, Cromohexal®
Levocabastine HCl
Microsuspension
Allergic rhinitis/antihistamine
Livocab®, Livostin®
Oxymetazoline HCl
Solution
Nasal decongestion/vasoconstrictor
Afrin®, Dristan®, Nasivin®, VicksSinex®
Beclomethasone diproprionate
Suspension
Allergic rhinitis/corticosteroid
Beconase®, Nasobec®,Qnasl® and generics
Fluticasone propionate
Suspension
Allergic rhinitis and nasal polyps/corticosteroid
Nasofan®, Flixonase®, Flonase® and generics
Momethasone furoate
Suspension
Allergic rhinitis and nasal polyps/ corticosteroid
Nasonex®, Mommox®
Budesonide
Suspension
Allergic rhinitis, rhinitis and nasal polyps/ corticosteroid
Rhinocort® spray
Rhinocort® turbuhaler
Powder
8.1.2 Systemic Action
Nasal administration of medicines is an effective way of systemic delivery of active substance, alternative to oral and intravascular delivery. Advantages of nasal systemic delivery include relatively large surface area available for absorption, rapid onset of therapeutic action, avoidance of first-pass metabolism (see Sect. 16.2.6), non-invasiveness of application of the active substance, resulting in patient comfort and compliance [8].
Generally speaking active substances with a systemic therapeutic action can be formulated as nasal preparations under the following conditions: high water-solubility (required dose must fit in 25–150 microlitres vehicle), sufficient chemical stability, no unpleasant smell or taste, favourable nasal absorption parameters, minimal nasal irritation and clinically important properties such as fast onset of therapeutic action, low dosage (normally less than 25 mg per dose), and no toxic metabolites [3, 8].
In preparations intended to obtain a systemic effect a spray solution is the favourite dosage form, because it enables accurate dosing. Examples of nasal preparations for systemic purpose are listed in Table 8.2. Examples of licensed preparations are nasal sprays with buserelin, fentanyl and vaccines (e.g. against airway infections) [8].
Table 8.2
Nasal preparations for systemic purpose (examples)
Active substance | Physical form | Indication/therapeutic class | Preparation |
|---|---|---|---|
Desmopressin | Solution | Central diabetes insipidus/derivative of the antidiuretic hormone | DDAVP, Minrin® |
Nafarelin acetate | Solution | Endometriosis/agonist of gonadotropin-releasing hormone | Synarel® |
Oxytocin | Solution | Gynaecological hormone (uterotonic, uterostiptic) | Syntocinon® |
Sumatriptan | Solution | Migraine/antimigraine agent | Imigran®, Imitrex®, Rosemig® |
Fentanyl citrate | Solution | Chronic pain/opioid analgesic | Instanyl®a, PecFent®a |
8.1.3 Advantages and Disadvantages of Nasal Preparations
The advantages and disadvantages of nasal preparations are summarised in Table 8.3.
Table 8.3
Advantages and disadvantages of nasal preparations. + the advantage applies for this type of preparation, × the disadvantage applies for this type of preparation
Nasal preparations with local effect | Nasal preparations with systemic effect | |
|---|---|---|
Advantages | ||
Administration by patient, at home | + | + |
Good adherence | + | + |
Accurate dosing possible | + (only for sprays) | + |
Little risk of overdosing | + (except for sprays in young children) | + |
High absorption, pharmacokinetic profile comparable with intravenous injection | – | + (for lipophilic substances) |
Fast absorption, onset of therapeutic effect within 30 min | – | + (for lipophilic substances) |
– | + | |
Possible alternative for active substances with low biological availability caused by insufficient absorption or extensive first-pass metabolism | – | + |
Possible induction of systemic or local immune response without injection (vaccines) | – | + |
Disadvantages | ||
Ciliotoxicity and nasal irritation by active substances or excipients | × | × |
Fast clearance (15–20 min) of liquids and powders due to activity of mucociliary apparatus | × | × |
Variablility in mucociliary clearance related to patient condition and environmental factors (moisture, temperature) | × | × |
Variability in absorption and therapeutic effect related to the nature of the active substance and condition of the patient | × | |
Nasal absorption of systemically acting substances resulting in a profile with ups and downs | – | × (when an even profile is needed) |
Limited volume that can be administered per nostril (25–200 microlitres) | (×) | × |
Accurate dosing not possible | × (nasal drops) | – |
Low biological availability of hydrophilic substances, such as peptides with a high (>1,000 Da) molecular weight | – | × |
Enzymatic degradation or metabolism on the mucosa | – | × |
8.2 Definitions
The European Pharmacopoeia (Ph. Eur.) defines nasal preparations as a liquid, semisolid or solid preparations intended for administration to the nasal cavities to obtain a local or systemic effect. They contain one or more active substances. Nasal preparations are as far as possible non-irritating and do not adversely affect the functions of the nasal mucosa and its cilia. Aqueous nasal preparations are usually iso-osmotic and may contain excipients, for example, to adjust the viscosity, to adjust or stabilise the pH, to increase the solubility of the active substance or to stabilise the preparation.
Nasal preparations are supplied in multidose or single-dose containers, provided, if necessary, with a suitable administration device, which may be designed to avoid contamination.
The Ph. Eur. lists
Nasal drops and liquid nasal sprays
Nasal powders
Semisolid nasal preparations (nasal ointments and gels)
Nasal washes
Nasal sticks
Nasal drops and liquid nasal sprays are solutions, emulsions or suspensions intended for instillation or spraying into the nasal cavities. Nasal powders or nasal insufflation powders are intended for insufflation into the nasal cavity by means of a suitable device. The size of the particles are such as to localise their deposition in the nasal cavity. In nasal sticks and so-called inhalation ointments mostly volatile active substances are formulated in a fatty base. Nasal washes are generally aqueous iso-osmotic solutions intended to cleanse the nasal cavity. If they are intended for application on injured parts of the mucosa, or prior to a surgical operation, they have to be sterile. Nasal powders and nasal sticks are not very common in pharmacy practice. Therefore they are not discussed in this chapter.
8.3 Biopharmaceutics
8.3.1 Anatomy and Function of the Nose
The nostril, the vestibulum nasi, is covered by hairy skin for 1–1.5 cm inward. The nose skin is not different from the rest of the skin, and can suffer from the same disorders. Further inward the skin is replaced by the nasal mucosa, which is covered by cilia (see Fig. 8.1).
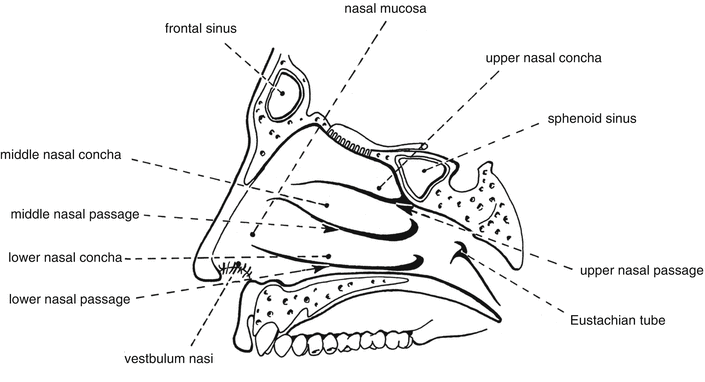

Fig. 8.1
Schematic cross section of the nose. Source: Recepteerkunde 2009, ©KNMP
The nasal epithelium contains cells with or without cilia, mucous cells and basal cells. The submucosa contains glands that produce mucus and an aqueous secretion. Nasal mucus consists of 95 % of water and contains 2 % mucine, 1 % inorganic salts, 1 % proteins (albumin, immunoglobulines, lysozyme) and less than 1 % of lipids. Mucus comes from the chalice-shaped mucous cells and the submucosal glands. The mucus layer (thickness 5 μm) actually consists of two layers. The lower part is a aqueous layer, in which cilia move. The upper layer is a discontinuous viscous mucus layer resting on the cilia, which is passed on by the cilia in the direction of the pharynx. The viscosity of this aqueous layer (sol layer) and gel layer, respectively, has influence on the mucociliary clearance. In the case of rhinitis the sol layer is so thick that the cilia cannot reach the upper layer (gel layer) to transport it to the pharynx. If the upper layer becomes too viscous due to dehydration, the cilia do not have sufficient power to move and clear it. The mucus layer has several different functions: it covers and protects the mucosa physically and by the action of enzymes, it has a capacity for water retention, allows the transfer of warmth and moves particles down to the nasopharynx [10, 11].
The thin, porous and highly vascularised nasal epithelium has a high total blood flow, which facilitates fast absorption of substances. Direct transport to the systemic circulation or the central nervous system makes it possible to obtain a rapid therapeutic effect. The intranasal absorption depends on the mucociliary clearance, pathological conditions such as infections, allergy and obstruction, mucus secretion, moisture content, enzymatic degradation, and blood flow. It should be remembered that the blood flow can be affected by either locally or systemically active substances. These phenomena can determine the nasal absorption of substances. Oxymetazoline and clonidine reduce the blood flow, while phenylephrine and salbutamol raise it.
8.3.1.1 Mucociliary Clearance
The function of the nose is, besides being the olfactory organ, to prepare the air in such a way that the airways and the lungs will not be damaged. In the nasal cavity the air is warmed up and moisturised before reaching the lungs. Coarse dust particles are held back by the hairs at the entrance of the nose, while smaller particles and micro-organisms can pass this first barrier, but are retained in the mucus layer. The cilia show a coordinated movement in wave-like patterns. By these movements the mucus with all the retained particles (dust, bacteria, powders, oil droplets) is drained to the pharynx, where the soft palate conducts it to the oesophagus by the swallowing movement. The coordinated movement (phase and frequency) is necessary for an efficient mucociliary clearance [11, 12].
8.3.1.2 Ciliary Beat
A well operating ciliary epithelium is important in prevention as well as cure of many diseases of the airways. The activity of the cilia depends on a number of factors, including temperature and humidity of the air, pH and viscosity of the mucus layer. Besides pathological conditions (allergic diseases, sinusitis, measles) also chemical influence may inhibit the action or even destroy the ciliary epithelium. This is called ciliotoxicity. Ciliotoxicity is an important reason to restrict the period of use of nasal preparations.
The inhibiting effect of anaesthetics on the ciliary epithelium is supposed to be an important cause of respiratory infections following surgery [12].
The rate of mucociliary clearance differs between individuals (fast and slow movers), but it does not depend on gender or age [11].
8.3.2 Biopharmaceutical Aspects of Nasal Preparations
Section 16.2.6 discusses biopharmaceutics of nasal preparations from a general biopharmaceutics viewpoint. This subsection adds some more specific details, first on the nasal absorption and then on the many investigations on absorption enhancing substances. The interest for nasal absorption is predominantly raised by the desire to find an alternative administration route for systemically acting active substances.
8.3.2.1 Intranasal Absorption
The mucociliary clearance rate may influence the intranasal absorption of systemically active substances. Pathological conditions and an accelerated rate of mucociliary clearance shorten the contact time between active substance and the absorbing mucosa. A delayed mucociliary clearance will have the opposite effect. Nasal hypersecretion dilutes the medicine solution and delays passive absorption. In addition it may lead to a local loss of some of the medicine due to a washout effect. A change in pH of the mucus layer may have consequences on the ionisation of some substances, and thus on their absorption [11].
8.3.2.2 Absorption Enhancers
Three ways exist to improve limited nasal absorption of systemically acting substances:
Using substances enhancing absorption through the mucosa
Using enzyme inhibitors to reduce degradation of active substances
Using mucoadhesive polymers, to make the preparation stay in the nasal cavity for a longer time
Any substance added to improve absorption should be pharmacologically inert, with no taste or smell, non-allergenic, non-irritating, non-toxic, affecting the structure of the mucosa and the mucociliary clearance only in a reversible way [11, 12]. Examples of mucoadhesive polymers are carbomers, chitosan and carmellose. They lengthen the residence time of nasal powders or suspensions in the nasal cavity [13, 16–18]. In addition to that carbomers bind in a reversible way with the tight junctions of the epithelium, thus facilitating paracellular transport [11, 12, 19–21]. Using cell culture and animal models, the mechanism of absorption enhancement of chitosan was also shown to be the transient opening of the epithelial tight junctions combined with the mucoadhesion [22]. However, more research has to be done on the effects of chronic use of mucoadhesive polymers in nasal preparations.
Absorption enhancers such as cyclodextrins can enhance the biological availability of intranasally administered medicines [23]. Research has mainly been focused on the influence of these excipients on systemic availability, but an enhanced local effect seems possible as well.
In short term studies cyclodextrins caused less histological changes of the nasal epithelium in rats than for instance benzalkonium chloride [24]. Other studies in rats showed that dimethyl betacyclodextrin, methylated betacyclodextrin and hydroxypropylbetacyclodextrin are safe and efficient enhancers of nasal absorption [8, 25, 26]. These results suggest that cyclodextrins (see also Sect. 18.1.4) can be used in nasal preparations, but more research is still needed.
Surfactants are also used to promote penetration of ingredients with systemic activity through the nasal mucosa. Their mechanism of action is based on a change of the permeability by disturbing (reversibly or irreversibly) the structural integrity of the mucosa. Polysorbate 80, for instance, has a strong negative effect on the cilia, which is however reversible.
A range of nasal peptides such as desmopressin, buserelin, nafarelin and oxytocin, have been formulated into licensed nasal products. However, none of them contains a nasal absorption enhancer. Even though these nasal products are characterised by low peptide bioavailability they are efficacious, as low systemic levels are needed to exert a therapeutic effect. However, developing of novel safe and efficient nasal absorption enhancers is of great interest to improve bioavailability of presently marketed peptides and to provide sufficient nasal permeability of less potent biologicals [22].
Several novel nasal absorption enhancer systems with promising preclinical or clinical data or both are now being commercially developed (i.e. cyclopentadecalactone, alkylsaccharides, chitosan, low methoxylpectin, hydroxyl fatty acid ester of polyethylene glycol) and have been reviewed in the literature [22]. Low methoxylpectin is already used in PecFent®, an intranasal preparation with fentanyl (Table 8.2). It contains the PecSys® delivery system, an in situ gelling system that gels due to interaction with calcium ions in the nasal fluid [29].
8.3.2.3 Local Effect
Not much is known about the biopharmaceutics of nasal preparations with a local effect. Dosing is done on a therapeutic result basis. What is known is that bioavailability and residence time are influenced by:
The position of the head during administration
The droplet size, which depends on different factors, including the interfacial tension of the solution and the dropper
The pattern of atomisation, that depends on the properties of the nozzle
The viscosity of the solution
The administered volume (number of drops or spray volume)
The site of deposition (anterior or posterior part of the nasal cavity)
Pathological situations and the condition of the mucosa (cold or flu, nose congestion, runny nose, nasal polyps, etc.), which affect absorption and mucociliary clearance
8.4 Adverse Effects and Toxicity of Nasal Drops and Sprays
As the mucosa is highly sensitive to irritation, nasal toxicity of active substances and excipients is an important issue in formulating nasal preparations, especially when they are intended for treatment of chronic diseases [11]. Nearly all substances used in nasal preparations have a negative influence on the ciliary beat, and are therefore ciliotoxic. The influence may vary from a temporary (reversible) effect up to an irreversible inhibition of the ciliary beat [30]. In many nasal drops and nasal sprays preservatives cause the toxic effect on cilia [31], but the active substance itself may also have a negative influence on the ciliary epithelium. Nasal drops with decongestants have been shown to exhibit relatively low ciliotoxicity (e.g. Xylometazoline nasal drops 0.025 %, 0.05 % and 0.1 % (see Table 8.4) as well as a number of licensed preparations) [32].
Xylometazoline hydrochloride | 0.025 g |
Benzalkonium chloride | 0.01 g |
Disodium edetate | 0.1 g |
Disodium phosphate dodecahydrate | 0.1 g |
Sodium chloride | 0.8 g |
Sodium dihydrogen phosphate dihydrate | 0.15 g |
Water, purified | ad 100 mL |
A review of the ciliotoxicity of other active substances that are used in nasal preparations, including local anaesthetics, antibiotics, antihistamines and corticosteroids can be found in the literature [12].
The administration of nasal drops or sprays may sometimes cause temporary irritation of the nasal mucosa. Systemic (side) effects, e.g. of decongestants, may be seen as a result of absorption by the nasal mucosa and the gastrointestinal tract. Problems of this kind can be avoided, if the patient carefully follows the instructions for use, the quantity to be administered and the duration of the therapy.
Especially in young children the use of nasal drops or sprays should be restricted. An overdose administered in the nose (e.g. by too high concentration) will more quickly lead to intoxication in young children than in adults, as the absorption surface of the mucosa compared to body weight in children is larger than in adults. In using nasal sprays, the contact absorption surface is larger than for nasal drops, so overdosing is more likely to occur. Due to the ease with which nasal sprays can be administered there is a real risk of overdosing in children. This risk led to the removal of the indication of primary nocturnal enuresis (PNE) in 2007 from all desmopressin nasal spray products, due to increased risk of hyponatremia and other adverse effects compared with the oral formulation [34].
In children under about 2 years of age there is the risk of a life-threatening laryngospasm. The nasal mucosa after a mechanical stimulus or a stinging smell can show a reflex apnoea or a spasm of the vocal cords. Menthol is a notorious example, but other volatile substances might cause the same reflex action.
In this section focus lies on the design of the formulation of nasal preparations, first for liquids and then for semisolid forms.
In addition to the active substance, nasal preparations often contain a number of excipients, including vehicles, buffers, preservatives, tonicity adjusting agents, solubilising agents, humectants, viscosity enhancing substances and possibly antioxidants. In the design of a nasal preparation, great care is needed when choosing a vehicle and other excipients. The integrity of the mucosal epithelium, the overall ciliary function and the mucus production should be retained as much as possible after administration. Otherwise the physiological function, and thus the protective action, of the nose will be disturbed. In many cases there will be a need to compromise between physiological requirements and restrictions with regard to the stability and pharmacological activity of the active substances.
8.5 Product Formulation
8.5.1 Liquid Preparations (Nasal Drops and Nasal Sprays)
8.5.1.1 Physico-chemical Properties of the Active Substance
A water soluble form of the active substance is preferred. This may bring about oxidation or hydrolysis reactions (see Sect. 22.2). Common sympathomimetics such as naphazoline, oxymetazoline and xylometazoline may hydrolyse in aqueous solution, but this happens only at a pH higher than what is normal for nasal preparations, or at a higher temperature than what is normal for the storage of these preparations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


