CHAPTER 25 Nonrespiratory Functions of the Lung
Although gas exchange is the primary function of the lung, the lung is also a major defense organ that protects the inside of the body from the outside world, and it is an important organ for metabolism. To cope with the inhalation of ubiquitous foreign substances, the respiratory system and, in particular, the conducting airways have developed unique structural features (e.g., the mucociliary clearance system), as well as specialized adaptive and innate immune response mechanisms. In addition, because the lung receives the total cardiac output, it is uniquely positioned to act as a metabolic regulator of venous blood before its entry into the systemic circulation. This chapter provides insight into mucociliary clearance and the immune defense systems of the lung, as well as describes the metabolic capabilities of the lung.
MUCOCILIARY CLEARANCE SYSTEM
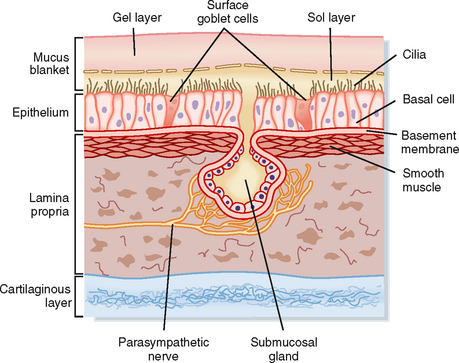
The mucociliary clearance system protects the lower respiratory system by trapping and removing inhaled pathogenic viruses and bacteria, in addition to nontoxic and toxic particulates (e.g., pollen, ash, mineral dust, mold spores, and organic particles), from the lungs. These particulates are inhaled with each breath and must be removed from the lungs. The three major components of the mucociliary clearance system are two fluid layers referred to as the sol (periciliary fluid) and gel (mucus layer) phases and the cilia, which are positioned on the surface of the airway epithelial cells (Fig. 25-1). The cilia are embedded in the periciliary fluid with only the tips of the cilia contacting the mucus. Inhaled material is trapped on the viscoelastic mucus, whereas the watery periciliary fluid allows the cilia to move freely. Effective clearance requires both ciliary activity and the appropriate balance of periciliary fluid and mucus.
PERICILIARY FLUID
The periciliary fluid layer is composed of nonviscous serous fluid, which is produced via active ion transport by the pseudostratified ciliated columnar epithelial cells that line the airways. Several mediators, under basal conditions and in response to inflammation, stimulate Cl− secretion by airway epithelial cells. The balance between Cl− secretion and Na+ absorption determines the volume and ionic composition of the periciliary fluid and maintains the depth of this fluid at about 5 to 6 μm (Fig. 25-1). When net NaCl transport into periciliary fluid is stimulated, diffusive entry of water (i.e., osmosis) into the periciliary fluid is enhanced because of the osmotic gradient that occurs transiently as a result of NaCl transport. Maintaining normal fluid depth and ionic composition in the periciliary fluid is important for rhythmic beating of the cilia and normal mucociliary clearance.
Mucus Layer
Cells That Produce Mucus
Four cell types contribute to the quantity and composition of the mucus layer: goblet cells, mucous cells, and serous cells within the submucosal tracheobronchial glands, as well as Clara cells. Goblet cells, also referred to as surface secretory cells, are present every five to six ciliated cells in the respiratory epithelium. They can be found up to the 5th tracheobronchial division and disappear beyond the 12th division. In many diseases, goblet cells appear further down the tracheobronchial tree, thus making the smaller airways more susceptible to obstruction by mucus plugging. Goblet cells secrete neutral and acidic glycoproteins rich in sialic acid in response to chemical stimuli. In the presence of infection or cigarette smoke or in patients with chronic bronchitis, goblet cells can increase in size and number, and they secrete copious amounts of mucus. Injury and infection change the properties of the mucus secreted by goblet cells by increasing its viscosity.
Submucosal tracheobronchial glands are present wherever there is cartilage in the upper regions of the conducting airways, and they secrete water, ions, and mucus into the airway lumen through a ciliated duct. The secretory cells of the submucosal gland include mucous cells located near the distal end of the duct and serous cells located at the most distal end of the duct. Although both cell types secrete mucus, their cellular morphology and mucus composition are distinctly different (Table 25-1). Mucous cells secrete acidic glycoproteins, whereas serous cells secrete neutral glycoproteins and bactericidal compounds, including lysozyme, lactoferrin, and antileukoprotease. Submucosal glands increase in number and size and can extend to the bronchioles in diseases such as chronic bronchitis (i.e., inflammation of the bronchi). This leads to increased mucus production, alterations in the chemical composition of the mucus (i.e., increased viscosity and decreased elasticity), and the formation of plugs that are manifested clinically as airway obstruction. Mucus secretion from submucosal tracheobronchial glands is under parasympathetic (cholinergic), sympathetic (adrenergic), and peptidergic (vasoactive intestinal polypeptide) neural control. Local inflammatory mediators such as histamine and arachidonic acid metabolites also stimulate mucus production.
Table 25-1 Properties of Submucosal Gland Cells
| Serous Cells | Mucous Cells | |
|---|---|---|
| Granules | Small, electron dense | Large, electron lucent |
| Glycoproteins | Acidic | |
| Hormones | α- > β-Adrenergic | β- > α-Adrenergic |
| Receptors | Muscarinic | Muscarinic |
| Degranulation |
Cilia
There are approximately 250 cilia per airway epithelial cell, and each is 2 to 5 μm in length. Cilia are composed of nine microtubular doublets that surround two central microtubules held together by dynein arms, nexin links, and spokes. The central microtubule doublet contains an ATPase that is responsible for the contractile beat of the cilium. Cilia beat with a coordinated oscillation in a characteristic, biphasic, and wavelike rhythm called metachronism. They beat at approximately 1000 strokes/min, with a power forward stroke and a slow return or recovery stroke. During their power forward stroke, the tips of the cilia extend upward into the viscous mucus layer and thereby move it and the entrapped particles. On the reverse beat, the cilia release the mucus and withdraw completely into the sol layer. Cilia in the nasopharynx beat in the direction that propels the mucus into the pharynx, whereas cilia in the trachea propel mucus upward toward the pharynx, where it is swallowed.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree