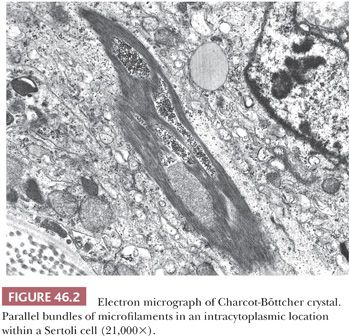
On ultrastructural examination, adult Sertoli cells have convoluted nuclei and prominent nucleoli. The basal cytoplasm contains lipid droplets, glycogen, spherical and elongated mitochondria, thin filaments, and a Golgi complex. Charcot-Böttcher crystals, elongated bundles of parallel microfilaments that measure up to 25 µm in length, 1 µm in width, and extend in various directions, may be present within the cytoplasm and may sometimes be large enough to be seen on light microscopy (Fig. 46.2). Ultrastructurally, the crystals appear to merge with vimentin-type intermediate filaments, both of which are known to be increased in cryptorchid testes and testes with Sertoli cell–only syndrome (germ cell aplasia) (4). The functions of the Sertoli cell have been increasingly recognized; it has been shown to play a central role in the development of the mature testis and thus male phenotype. It is the first recognizable cell in the indifferent fetal gonad, and its appearance allows for seminiferous cord formation, differentiation and functional activity of Leydig cells, and prevention of germ cell entry into meiosis (5). Sertoli cells secrete anti-müllerian hormone (AMH), leading to regression of the müllerian ducts (5,6). After puberty, these cells switch to a supportive role in spermatogenesis. This switch from an immature, proliferative cell involved in testicular differentiation to a mature, nonproliferative cell involved in support of spermatogenesis is a dramatic one, with both functional and morphologic differential features. Fetal Sertoli cells have elongated nuclei with inconspicuous nucleoli, as opposed to the mature cell. At puberty, tight cell junctions form that define the blood–testis barrier, creating an outer basal compartment containing spermatogonia and preleptotene primary spermatocytes and an inner adluminal compartment in which more mature forms reside and where meiotic and postmeiotic steps of spermatogenesis take place.
Variable protein profiles that can be identified immunohistologically have been noted depending on the maturational state of the Sertoli cell. It has been demonstrated that Sertoli cell–only tubules sometimes express markers of immature cells (AMH, CK18, and M2A antigen) while lacking antigens such as androgen receptor (AR), which are typical of mature Sertoli cells (7).
Orth et al. (8) have shown in rats that the number of Sertoli cells present determines both testicular size and daily sperm production. Although showing wide variability among men, Johnson et al. (9) showed this is also true in the adult human testis. Measurement of serum inhibin B, one of the Sertoli cell products, has shown some promise in assessing Sertoli cell function (10).
Human Sertoli cells in cell culture secrete androgen-binding protein (ABP) and are responsive to follicle-stimulating hormone (FSH) (11). ABP is a secretory protein with a high affinity for testosterone and dihydrotestosterone. Once an androgen–ABP complex is formed, it is transported to the epididymis. Other substances synthesized by the Sertoli cell include inhibin and activin subunits (α- and β-subunits). Inhibin exerts negative feedback on gonadotropin-releasing factor and FSH release by the hypothalamus and anterior pituitary, respectively, whereas activin exerts positive feedback on FSH release (1).
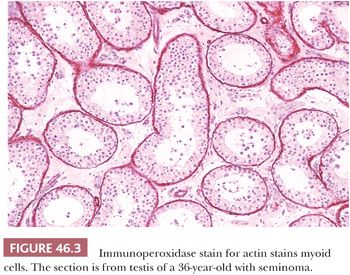
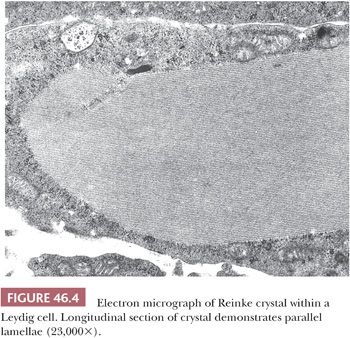
The tubules are lined by a basal lamina, peripheral to which are layers of myoid cells that stain positively for desmin, muscle-specific actin, and vimentin with immunohistochemical (IHC) stains (Fig. 46.3). Leydig cells, which produce testosterone after puberty, lie in the loose connective tissue between the seminiferous tubules. These cells are variably shaped, often polyhedral, and are found in proximity to blood vessels. They measure about 20 µm in diameter and contain nucleolated round nuclei and eosinophilic, sometimes vacuolated, cytoplasm. Within the cytoplasm are varying amounts of brown lipochrome pigment. In the postpubertal testis, some Leydig cells contain eosinophilic crystals of Reinke, which may be seen in long or cross section and have a crystalline ultrastructural appearance (Fig. 46.4).
EMBRYOLOGY
At the time of conception, fertilization of an X-bearing egg by a Y-bearing sperm determines the formation of the testis. The genetic basis for human femaleness and maleness was recognized in 1923 to be the chromosomal pairings of XX and XY, respectively. Initially, it was thought that the sex-determining factor was the presence of two X chromosomes, but since 1959, it has been understood that it is the Y chromosome that contains the testis-determining factor (TDF). Various candidate genes have been examined, and the one currently believed to be the TDF is Sry (sex-determining region of Y), a member of the Sox family of transcription factors (12). By day 24, primordial germ cells are recognizable in humans by their large size and alkaline phosphatase content in the endodermal layer of the yolk sac. Under the probable influence of chemotactic factors and connected to each other by cytoplasmic processes, the germ cells then migrate along the dorsal mesentery of the hindgut to the newly appearing genital ridges. One clinical consequence of faulty migration of the primordial germ cells to extragonadal sites is the possible development of germ cell tumors along the track of migration. The genital ridges appear midway in the fifth week by proliferation of the coelomic epithelium and underlying mesenchyme along the medial border of the mesonephros. Primary sex cords develop in the genital ridges in the sixth week, under the influence of the transcription factor Sox-9. The sex cords enlarge, differentiate, and give rise to Sertoli cells, which, along with the migrating germ cells, form the seminiferous tubules. The Leydig (steroidogenic) cells, straight tubules, and rete testis arise from the surrounding mesenchyme. The rete testis eventually joins the efferent ducts and epididymis, derived from the mesonephric tubules. The sex cords become separated from the overlying surface by a layer of connective tissue called the tunica albuginea. It is during this development of the genital ridge and sex cords that the Sry transcript is identified in males. Germ cells are not required for the development of the testis. It appears that Sry acts by inhibiting the function of DAX-1, an X-linked member of the nuclear hormone receptor family and an inhibitor of testis formation (12).
Initially, Leydig cells develop in the embryonic testis at about week 8. They begin to secrete testosterone and androstenedione, which is important for the developing male genital duct system and external genitalia during weeks 9 to 14. These cells then begin to involute around weeks 17 to 18. They are still present, however, until about 4 months postnatally, when they disappear until their reappearance at puberty, when they begin to stimulate spermatogenesis. At about the same time the Leydig cells begin to secrete testosterone, the Sertoli cells secrete AMH, which causes involution of the precursors of the female duct system (12).
POSTNATAL DEVELOPMENT
During the first 3 months of life, there is a hormonal surge referred to as minipuberty in which gonadotropins, testosterone, and inhibin A levels rise. This is associated with increased numbers of Leydig cells and Sertoli cells. Germ cell numbers also increase transiently to a maximum at 3 months. Berensztein et al. (13) compared proliferation and apoptotic rates in seminiferous tubules and interstitium during the neonatal period and later (young infants from 1 to 6 months and young boys 1 to 6 years of age) and found that the vigorous growth during the neonatal period may be mediated by decreased apoptosis. In the next 2 months, there is a fall in serum gonadotropins and testosterone to the typical low levels of childhood. Inhibin B is slower to fall (at approximately 15 months). It is thought that this transient stimulation of the hypothalamic–pituitary–gonadal axis is essential for the final transformation of gonocytes (primordial germ cells, which are mitotically active) into type A dark spermatogonia (14). Histologically, during the newborn period, mostly aluminal tubules contain numerous immature Sertoli cells and rare fetal spermatogonia. Prepubertal Sertoli cells are characterized by scant cytoplasm and round nuclei with inconspicuous nucleoli.
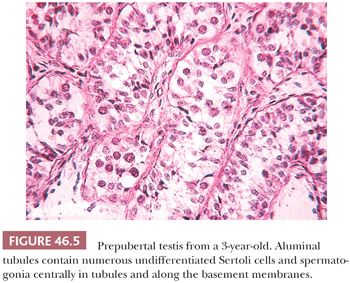
Although earlier studies indicated that testicular development is static during the first years of life (15), stereologic studies have shown progressive increase in testicular volume from birth to adulthood (16). This increase is not sufficient during the first 5 or 6 years to produce distinctive changes by light microscopy (Fig. 46.5). In Müller and Skakkebaek’s study (16), mean tubular diameter was constant through year 5, with slight enlargement through year 14 and a significant increase in diameter in the tubules of 14- to 17.9-year-olds. The numeric density of germ cell nuclei per tubule increased progressively from age 5 years, as did the total number of germ cells per boy. Seminiferous tubules occupied about half the testicular volume before puberty and about 67% from age 14 to 17.9 years. By 8 years, primary spermatocytes may be evident. Development becomes progressive during puberty. Sertoli cells become more mature, with increased eosinophilic cytoplasm and oval nucleolated nuclei. Mitotic figures are not seen in Sertoli cells. Spermatogenesis and spermiogenesis may be seen by year 11. Leydig cells reappear as soon as 8 years and are more numerous at 11 through 14 years. They have been described ultrastructurally in boys 3 through 9 years and may be present earlier (17). During puberty, the seminiferous tubules enlarge as a result of spermatogenesis and the acquisition of a tubule lumen. Once these changes occur, it is not possible to determine age histologically.
Changes related specifically to advancing age are not readily discernible in an individual testis and there is no one feature that characterizes the senescent testis. After age 40 years, serum luteinizing hormone (LH) and FSH increase, and testosterone slowly decreases (18). Weights of testes are similar in young and older men, but the percentage of total testicular weight represented by the tunica albuginea and the thickness of the tunica albuginea are greater in older adults. Weight and cellular composition of the testes vary among Caucasians, Hispanics, and Asians (19). Although the weight of testicular parenchyma may be either similar to younger men or reduced with aging, the daily sperm production per testis and per gram of testis is less than in young men (20). Germinal cell degeneration occurs in the testes of both younger and older men during spermatogonial mitosis and meiosis as well as during spermiogenesis. Additional degeneration occurs during meiosis of older men. Older men show reductions in the number of Leydig cells and non-Leydig interstitial and myoid cells (21–23). However, there has been controversy regarding Leydig cell numbers; Honoré (24) described Leydig cell hyperplasia with aging, but this was not assessed in a quantitative fashion. He also described tubular sclerosis, focal mononuclear cell infiltration, capsular smooth muscle hyperplasia, and dilation of the rete testis.
In the human, in general, spermatogenic and hormonal function do not abruptly cease. It is difficult to determine causes of the various changes, but decrease in hormonal function is probably at least partly due to a loss of about 80 million Leydig cells per decade (22). Tubular sclerosis occurs in testes of men with arteriosclerosis, but the fact that arteriosclerosis specifically causes the changes remains to be proved (25). Reduced function and altered structure of the testis in older men have multiple etiologies, including reduced nutrition and intercurrent disease.
CONGENITAL ABNORMALITIES
CRYPTORCHIDISM
Cryptorchidism (undescended testis) has an incidence of 2% to 8% in full-term males, decreasing due to spontaneous descent to 1% to 2% of all boys at 3 to 12 months. This condition is associated with decreased fertility and a three- to fivefold increased risk of testicular cancer (26–29). Thus, this congenital abnormality may be encountered by surgical pathologists in specimens containing tumor or testicular tissue obtained at the time of orchiopexy or repair of torsion.
The etiology of testicular maldescent is being increasingly understood, and its causes include abnormalities in the hypothalamic–pituitary–testicular axis, abnormal testicular differentiation, deficient androgen production or action, deficient production or action of AMH, or deficient action of insulin-like hormone 3 (INSL3). A recent study has also suggested a role of the Wnt signaling pathway: As Wnt-5a and its receptor Ror2 colocalize in the rat gubernaculum, Wnt-5a knockout mice fail to undergo gubernacular swelling, similar to INSL3 knockout mice, and a rare syndrome in humans (Robinow syndrome) caused by mutations in either Wnt-5a or Ror2 is associated with a high frequency of cryptorchidism (30). Embryologically, descent of the testes occurs in two main phases: transabdominal, which in animals, at least, requires INSL3; and inguinoscrotal, which requires androgens. In humans, it is usually the inguinoscrotal phase that is disturbed; in only 5% of surgically repaired cases is the testis intra-abdominal (31). Retractile testes appear to be more likely to spontaneously descend or to descend after hormonal therapy (32). However, this may not be the innocuous condition previously thought because there has been evidence that, at least in some retractile testes, degenerative changes occur along with decreased numbers of spermatogonia (33).
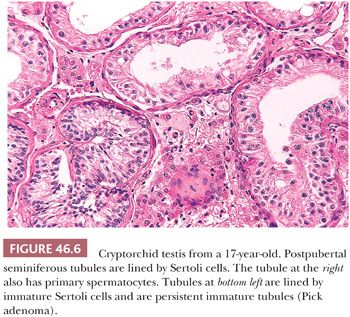
In pathologic specimens, various histologic findings have been reported which vary with age, testicular position, and possibly prior hormonal therapy. Huff et al. (34) described fewer germ cells per seminiferous tubule after the first year of life in the cryptorchid testis than in the normally descended testis. Nistal et al. (35) classified prepubertal undescended testes into four groups according to tubular diameter, tubular-fertility index (TFI: number of tubules with germ cells), Sertoli cell index (SCI: number of Sertoli cells per transverse tubular section), and the mean tubular diameter (MTD). The most severely involved testes showed reduced TFI, reduced SCI, and decreased MTD. These authors believed that orchiopexy improves the chance for fertility only in those cases with minimal alterations (i.e., slight tubular hypoplasia). The more severely affected prepubertal testes will develop postpubertal hypospermatogenesis, maturation arrest, and germinal cell aplasia or hypoplasia (Fig. 46.6). In a later study, Nistal et al. (36) categorized progression of histologic abnormalities in 21 men who had biopsies at the time of orchiopexy and later when they had biopsies for infertility as adults. Using the parameters mentioned earlier, this study showed that less severe changes in the infant testis at the time of orchiopexy usually correlated with better spermatogenesis in adulthood.
Tunica propria thickening of the seminiferous tubules is another histologic finding seen in cryptorchidism, which has been found to develop as early as the first year of life (37). After puberty, histologic changes become progressively more apparent and, in addition to the tunica propria thickening, include reduced tubular diameter, tubular sclerosis, interstitial fibrosis, and more prominent Leydig cells. Nistal et al. (38,39) also described oncocytic changes, vacuolation, and athrocytosis (apical granules) in Sertoli cells of retractile testes, which were subsequently found in testes with germinal cell aplasia, macro-orchidism, interstitial orchitis, acquired immunodeficiency syndrome (AIDS), male pseudohermaphroditism (46,XY disorder of sex development), and in a testis associated with varicocele.
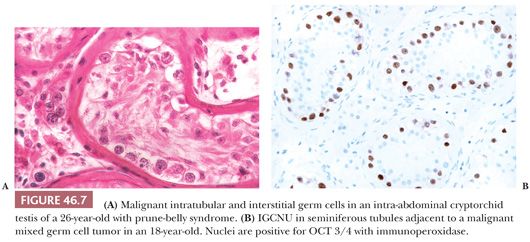
In addition to the findings described earlier, it is particularly important that biopsies of cryptorchid testes be examined for the presence of intratubular germ cell neoplasia (ITGCN), which is found frequently in association with germ cell tumors (Fig. 46.7A). However, this finding occurs only rarely in the prepubertal undescended testis. The malignant cells of ITGCN may be very few and can be missed. Immunoperoxidase stains for placental alkaline phosphatase (PLAP), CD117, OCT3/4, SALL4, or podoplanin can be particularly helpful in identifying these neoplastic cells (Fig. 46.7B). Germ cell tumors of almost any type may develop in the undescended testis, with the notable exception of spermatocytic seminoma. In a recent study of 16,983 men, the relative risk of testicular cancer in patients undergoing orchiopexy before the age of 13 years was 2.23, compared to 5.4 when the procedure was done at 13 years or older (40).
Undescended testes of postpubertal individuals are unlikely to be capable of fertility without the use of assisted reproductive techniques. However, men with previous unilateral cryptorchidism and orchiopexy have a paternity rate statistically equal to a control group (41). Undescended testes are more susceptible to torsion and infarction. When torsion occurs in the undescended testis of an adult, the gonad is at risk for harboring a malignant germ cell tumor. Gross examination of the organ should be carefully performed, and any scars, infarcts, or nodules should be noted. Scars or infarcts may be the site of regressed tumors, and small nodules of approximately 1-mm diameter may represent clusters of persistent immature tubules, so-called Pick adenomas or tubular congeries (Fig. 46.6). Patients with metastatic germ cell neoplasms may have a testis removed in an attempt to discover and treat the primary tumor. In this situation, if the neoplasm is not obvious, the entire testis should be sectioned and submitted, whether the testis is undescended or normal in position. Balzer and Ulbright (42) have described clinical, gross, and histologic testicular findings supportive of a diagnosis of a regressed (“burnt-out”) testicular germ cell tumor. Specifically, burnt-out germ cell tumors show circumscribed to irregular foci of scarring and testicular atrophy as well as a variable frequency of the following findings in the scarred areas: lymphoplasmacytic infiltrates, “ghost” tubules, angiomatous foci, siderophages, and coarse intratubular calcifications. Findings in the surrounding testis included ITGCN, Leydig cell prominence, necrosis, and tubular microliths (rarely within the scar).
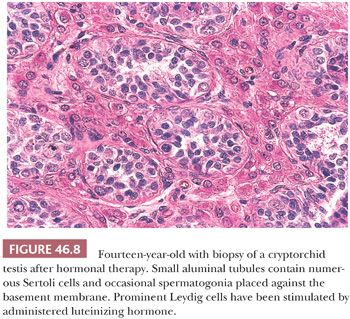
The trend has been for surgeons to treat cryptorchid patients earlier, not only because of the risk of developing malignancy but also in the hope of maintaining fertility. Previously, surgery was sometimes done after a trial of human chorionic gonadotropin or gonadotropin-releasing hormone (GnRH). It has been noted that this hormonal treatment is not very effective and may even cause increased apoptosis, leading to subsequent defects in spermatogenesis (43). Biopsies after such therapy in the first years of life have been shown to contain clusters of stimulated Leydig cells that ordinarily would have regressed (Fig. 46.8). Current recommendations for time of orchiopexy have ranged from doing the procedure before 1 year to prior to 15 to 18 months of age (28,43).
ANORCHIA
Anorchia is a rare occurrence in males clinically thought to have cryptorchid testes. In a phenotypically normal male, if the vas deferens is present, there had to have been a functioning ipsilateral testis during gestation. There are two reasons for failure to discover the gonad at the time of surgical exploration: either it does not exist or the urologist failed to find it. In the former circumstance, the gonad will have disappeared sometime after induction of the wolffian duct system, regression of the müllerian duct, and formation of external genitalia (approximately week 16 of gestation) (44,45). This is an example of testicular regression syndrome (see later in this chapter) and may be due to a variety of causes, including intrinsic gonadal disorder, infection, trauma, torsion and infarct, or prenatal hormone–induced atrophy resulting from overproduction of androgens (44). When regression occurs before seminiferous tubule and Leydig cell formation, there is ipsilateral absence of the wolffian duct system and failure of regression of the müllerian duct system.
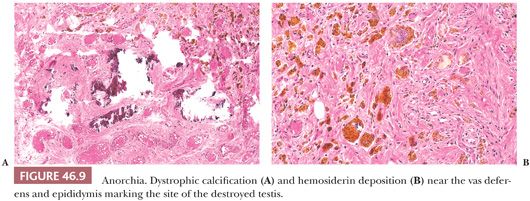
The urologist may call on the pathologist in the course of surgical exploration to determine whether testicular tissue is present. Fibrosis, hemosiderin deposition, calcification, or Leydig cells near the epididymis or proximal portion of the vas deferens indicate the position of the former testis (Fig. 46.9). When frozen section shows only fat, connective tissue, or epididymis, a separate intra-abdominal testis cannot be excluded (46,47). All tissue should be examined histologically, including multiple levels. The presence of vas deferens or epididymis, or both, and histologic changes should be noted, and it should be indicated that this is the probable location of the former testis. The presence of venous structures next to the vas deferens or epididymis should be noted because a testis probably cannot be present without a gonadal vein (48). The contralateral descended testis is usually enlarged in an individual with monoorchism.
POLYORCHISM
Polyorchism presents as an abnormal intrascrotal or intra-abdominal structure suggestive of tumor, maldescent, or torsion (44). The testes may each have an individual epididymis and vas deferens that join distally, a single epididymis that joins both testes and leads to a single vas deferens, or discordant connection of the excurrent duct system to the testes (45). The spermatogenic function of the testes is variable. Tumors have rarely been reported in polyorchism (45).
SPLENOGONADAL FUSION
In the first review of this entity, Putschar and Manion (49) classified fusion as being continuous, with the testis attached to the spleen by a fibrous band with or without adherent splenic tissue; or discontinuous, with the separate intrascrotal splenic nodules attached to the spermatic cord, epididymis, appendices of the epididymis or testis, or the testis itself. Splenogonadal fusion occurs mostly in males (95%), but the incidence in females may be underreported due to the relative inaccessibility of the ovaries for examination. It is a rare congenital anomaly with unclear etiology, which is assumed to occur between weeks 5 and 8 of gestation. The accessory spleen is usually found within the tunica vaginalis with a distinct capsule. Some cases (usually the continuous variant) are associated with other congenital anomalies, especially limb defects and micrognathia (50,51). A variety of changes may be present in the testis, including atrophy and fibrosis. In a case reviewed by one of the previous authors (HSL), seminiferous tubules adjacent to splenic tissue showed focal germinal cell aplasia and hypoplasia. Ando et al. (51) reported a case of left-sided splenogonadal fusion with contralateral testicular aplasia. Testicular germ cell tumors have been rarely reported in association with splenogonadal fusion (52).
Preoperatively, the patient may be interpreted as having a testicular neoplasm, although other presentations, including incidental finding during orchiopexy or inguinal hernia repair, bowel obstruction, and traumatic rupture of the splenic tissue, have been reported. The intrascrotal lesions may enlarge and become symptomatic as a result of circumstances that produce splenomegaly, such as infection (49). Recognition of this entity may prevent unnecessary orchiectomy during exploration for scrotal swelling because the ectopic spleen can usually be dissected off the gonadal structures with sparing of the testis.
ADRENAL CORTICAL RESTS
Rests of adrenal cortex occur in the testis, adjacent to the testis, or in the spermatic cord and epididymis in 4% to 15% of individuals (53). The rests are small, rounded, yellow structures composed of adrenal cortical tissue. No adrenal medullary tissue is present in the cortical rests. These rests are inconsequential except in cases of congenital adrenal hyperplasia (CAH) and Nelson syndrome. In CAH, multiple masses histologically similar to Leydig cell tumors occur in the testes and peritesticular areas. In cases of Nelson syndrome that have an adrenocorticotropic hormone (ACTH)–producing pituitary tumor, similar bilateral masses occur after bilateral adrenalectomy; because the source of ACTH production is neoplastic, ACTH is not suppressed by administered corticosteroids. In the case reported by Johnson and Scheithauer (53), nodules of eosinophilic cells of probable adrenocortical origin were present in both testes and the left spermatic cord. In the orchiectomy specimens, the testicular parenchyma adjacent to the intratesticular masses was devoid of Leydig cells because of absent LH secretion resulting from irradiation and surgical resection of the pituitary. However, irradiation failed to control the ACTH-producing pituitary adenoma. Regression of the native Leydig cells in this case further suggests origin of the testicular tumors to be ACTH-sensitive cells of probable adrenocortical origin. In both CAH and Nelson syndrome, the masses tend to be multifocal and bilateral, in contrast to Leydig cell tumors which may be histologically similar but rarely bilateral. Additional features helpful in distinguishing adrenal cortical rests from Leydig cell tumors include fibrous bands that separate the eosinophilic cells and a lack of crystals of Reinke. These masses may be detectable by ultrasonography.
ACQUIRED ABNORMALITIES
TORSION AND INFARCTION
Torsion of the spermatic cord may occur within the tunica vaginalis or external to it, so-called intravaginal or extravaginal torsion. Most cases of torsion occur intravaginally and result from high insertion of the tunica vaginalis on the spermatic cord. In this circumstance, the testis is not attached to the scrotal wall posteriorly and is suspended on a longer-than-normal spermatic cord, the so-called bell-clapper abnormality. Intravaginal torsion—the complete or incomplete twisting of the cord within the tunica vaginalis—generally occurs in adolescents and young adults. Torsion may be greater than 360 degrees. Torsion that lasts less than 6 hours probably will not cause a testicular infarct. If torsion lasts longer than 24 hours, the testis almost certainly will infarct (54). Thus, testicular torsion should be diagnosed and treated promptly. Torsion is a direct cause of infertility when it results in infarct or atrophy. Even if the testis is judged not to have been infarcted and is treated with orchiopexy, there is a substantial possibility that atrophy may ensue. Thomas et al. (55) studied 54 patients 4 years (mean elapsed time) after torsion and orchiopexy and found 45 to have atrophy of the affected testis. The degree of atrophy correlated with the duration of torsion. Patients who had torsion for more than 10 hours had a 50% decrease in testicular volume. Most patients with a history of unilateral torsion and orchiopexy had an abnormal semen analysis at the time of study, which might have resulted from an immunologic event at the time of torsion, from subclinical torsion of the nonorchiopexed testis, or from an abnormality of the contralateral testis unrelated to the occurrence or therapy of torsion. Hadziselimovic et al. (56) found increased apoptosis of primary and secondary spermatocytes in the biopsies of the contralateral testes obtained at the time of surgery to alleviate testicular torsion. Results in rats suggest that dehydroepiandrosterone (DHEA) may serve as a protective agent in preventing oxidative-type biochemical injury and the subsequent histologic changes seen with testicular torsion (57).
The pathologist usually is not part of the preoperative diagnostic or operative treatment of the patient; a frozen section is requested only when viability of the testis is in doubt. With present techniques, frozen section can usually determine whether testicular parenchyma has been infarcted. If the frozen section is quickly fixed with alcohol and acetic acid, lysis of red cells may diminish the appearance of interstitial hemorrhage.
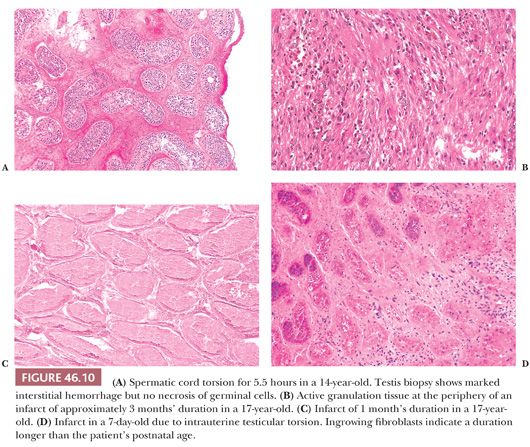
A cavalier approach to completely studying testes excised after torsion is not prudent. It is not usually possible grossly to determine the degree of torsion in such specimens, and sections are usually taken merely to verify the presence of infarct without carefully analyzing the spectrum and location of changes. Thus, sections of testis often do not help us to determine the duration of infarction. In cases studied by a previous author (HSL) from available biopsy and orchiectomy specimens, histologic changes have been noted that may be useful in determining the duration of the torsion. Up to 6 hours after torsion, the testis shows venous congestion and interstitial hemorrhage. A biopsy at 5.5 hours showed no nuclear pyknosis (Fig. 46.10A). At 9.5 hours, polymorphonuclear leukocytes (PMNs) were in the walls of capillaries, and there was severe interstitial hemorrhage but no definite infarct. At 4 days, there was hemorrhagic infarction and coagulative necrosis of testicular parenchyma with a PMN infiltration. Infarct and granulation tissue were present in specimens obtained 1 and 2 months after onset of torsion (Fig. 46.10B). Thus, infarcts of 4 days to 2 months showed complete infarction (Fig. 46.10C). If a section is not taken at the periphery of the infarct, granulation tissue will not be recognized (Fig. 46.10D). We have seen mummification of a testis that had been atrophic for 23 years. Fibrosis and calcium deposition also may occur in ancient infarcts.
Testicular pain simulating torsion may result from other causes. The small vessel vasculitis of Henoch-Schönlein purpura may necessitate a scrotal exploration but, with the urologist’s observation that the testis is grossly normal, usually no tissue is removed (58,59). Torsion of the appendix testis may mimic testicular torsion and result in a specimen of appendix testis that has undergone torsion with or without infarction. Segmental infarction may occur secondary to vasculitis, embolism, sickle cell disease, or sickle cell trait (60,61).
Although bilateral torsions occur rarely, it is good surgical practice to anchor the testis that has not undergone torsion to prevent it from doing so. In general, extravaginal torsion occurs in infancy and is rarer than intravaginal torsion. Recently, Kao et al. (62) postulated that chronic intermittent torsion can cause testicular intraparenchymal hemorrhage and/or necrosis that can clinically and radiographically mimic a mass. In all of the 30 cases they studied, they found associated vasculopathic changes in testicular and paratesticular vessels, specifically variable combinations of thrombi, intimal and medial fibrosis, fibrinoid necrosis, and vasculitis (62).
VARICOCELE
Varicocele is an abnormal dilation and tortuosity of veins in the pampiniform plexus of the spermatic cord. It is probably caused by incompetency of venous valves; it involves the left side in 90% of cases and is bilateral in about 10% (63). Varicocele involving the right side exclusively is extremely rare and may be associated with situs inversus, venous thrombosis, or venous compression resulting from a space-occupying mass (64). The negative effects, however, appear to be bilateral. Most men with varicoceles are able to conceive without intervention; however, this lesion is still the most common abnormality in men being evaluated for infertility. Although numerous theories have been advanced regarding the pathophysiology of varicocele leading to abnormalities in spermatogenesis, it is thought to be the result of elevation of scrotal temperature via reflux of abdominal blood. Several controversies exist regarding if and when to initiate surgical management (i.e., ligation or occlusion of the left spermatic vein). Most physicians dealing with male infertility believe that repair leads to increased fertility, but whether prophylactic surgery is warranted in adolescents and young men and the prognostic significance of varicocele size are still debated. Current available evidence strongly supports the conclusion that varicoceles do cause progressive deleterious effects on testicular function (volume of testes and semen production), at least in adolescents (65). In contrast, these effects over time probably do not occur in adult males. Varicocele size does seem to have prognostic significance and correlates with total motile sperm count. The recommendation of Diamond et al. (66) was to follow sperm count parameters in adolescents who initially show volume differentials between testes of greater than 10% but have more than 20 million sperm/mL.
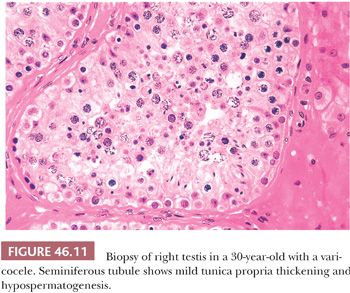
The pathologist rarely examines a varicocele or a large portion of one. Usually, a cross section of spermatic vein shows only variable thickening of the vein wall with fibrosis. Testis biopsies may be obtained at the time of high ligation or balloon occlusion of the left spermatic vein. Biopsies of either testis will show similar nonspecific morphologic changes consisting of decreased spermatogenesis with sloughing of immature germinal cells, degeneration of germ cells, and increased numbers of Leydig cells (Fig. 46.11). Agger and Johnsen (67) used a scoring system and found improvement in testicular histology 1 year after venous ligation. Andres et al. (68) demonstrated fibrosis of veins with narrowing of their lumens in two of six patients in addition to changes of hypospermatogenesis. Jones et al. (69) described changes in 13 adolescents that included peritubular sclerosis, small-vessel sclerosis, marked premature germ cell sloughing in more than 50% of tubules, and reduced numbers of germ cells. Varicocelectomy probably does not improve semen quality in men with Y chromosome microdeletions or karyotypic abnormalities (70).
MICROLITHIASIS
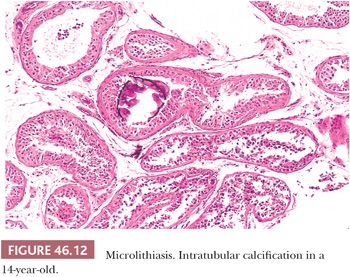
Testicular microlithiasis (TM) refers to microcalcification in seminiferous tubules (Fig. 46.12). Classic TM is discovered on an ultrasound examination and consists of more than five foci of calcification, each less than 2 mm in diameter, randomly distributed in one or both testes. TM has been recorded in children, adults, infertility patients, in testes harboring germ cell tumors, and in cryptorchid testes. The relative risk of testicular neoplasms in the presence of TM was reported as 13.2. Many of the patients in this study presented with testicular masses, but testicular germ cell tumors have rarely occurred after the ultrasound diagnosis of TM (71). Although there is an association of TM and testicular germ cell tumors, there is a marked discrepancy between the incidence of TM in a healthy adult population (5.6%) and the low incidence of testicular germ cell tumors in the general population (72), suggesting that the large majority of cases of microlithiasis are not associated with germ cell malignancy. Still, some authors consider it to be a possible predisposing factor or indirect indicator of premalignant disease. The current available data suggest that men with incidental TM (without other conditions such as Klinefelter syndrome, male pseudohermaphroditism [46,XY disorder of sex development], Down syndrome, subfertility or infertility, cryptorchidism, hypogonadism, fragile X syndrome, pulmonary microlithiasis, which themselves are risk factors for germ cell tumors) are at low risk for the development of germ cell tumors. Regular testicular self-examination is recommended in patients without risk factors (73,74). Clinical follow-up, including serial ultrasonography, is suggested in patients with risk factors for testicular germ cell tumors. Microlithiasis has been described in the rete testis, efferent ducts, epididymis, and vas deferens (75).
TESTICULAR INVOLVEMENT IN SYSTEMIC DISEASE
VASCULITIS
Systemic vasculitis frequently involves the testes (76). Testicular vasculitis is uncommon and may herald systemic disease; it should therefore be included in the differential diagnosis of testicular pain, swelling, and even palpable nodules. Imaging studies may not distinguish vasculitis from neoplasia, and definitive diagnosis requires examination of histologic material. The most common type of vasculitis in the testis is polyarteritis nodosa (PAN), which affects small- and medium-sized arteries and secondarily adjacent venules and arterioles. PAN may be associated with hepatitis B or human immunodeficiency virus (HIV). Although 60% to 86% of cases may have testicular involvement, only 20% have symptoms localized to the testis (76). Other testicular vasculitides include Wegener granulomatosis (77), Henoch-Schönlein purpura, giant cell arteritis, and various autoimmune connective tissue disorders such as systemic lupus erythematosus (SLE) (78). Occasional patients have isolated vasculitis of the testis or epididymis without systemic vasculitis, possibly triggered by local exposure to an agent capable of immunostimulation (79,80). Some cases of systemic vasculitis present as a testicular mass (81,82). Vasculopathic changes, including lymphocytic vasculitis and fibrinoid necrosis, have been reported in association with chronic intermittent torsion in patients without a history of systemic vasculitis and without subsequent development of systemic vasculitis, emphasizing the importance of clinical context and ancillary laboratory studies in assigning significance to isolated vasculitic changes in the testis (62).
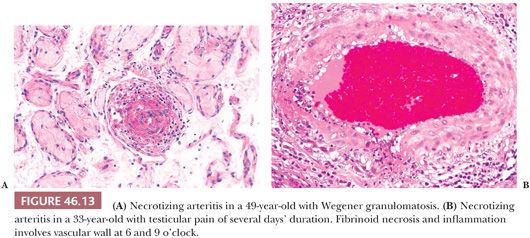
In a patient with possible systemic vasculitis, blind biopsy of an asymptomatic testis is unlikely to yield a positive diagnosis. The biopsy should be from a painful, enlarging, or shrinking testis and should consist of a wedge containing capsule, tunica vasculosa (the vascular zone beneath the tunica albuginea), and testicular parenchyma. In light of the focal nature of the lesions of vasculitis, the paraffin block should be serially sectioned if initial sections do not yield a diagnosis. Microscopically, vessel walls show inflammatory infiltrates with fibrinoid necrosis and intraluminal thrombi (Fig. 46.13). In Wegener granulomatosis, granulomatous inflammation is present. Biopsy of a testis with vasculitis might contain an infarct rather than vasculitis itself. Ischemic areas may also show edema, interstitial hemorrhage, or coagulative necrosis (76).
AMYLOIDOSIS
Systemic amyloidosis commonly involves the testis. Ozdemir et al. (83) found that 86% of patients with known amyloidosis had amyloid in the testicular biopsy. Amyloid deposition occurs in relation to blood vessels of the interstitium and, to a much smaller degree, in the walls of the seminiferous tubules. Seminiferous tubules with or without amyloid show reduced spermatogenesis. In examination of testicular specimens with marked hyaline thickening of the seminiferous tubules, the extent and texture of the hyaline change may suggest the differential diagnosis of amyloid. This diagnosis is unlikely unless there is extensive thickening of interstitial blood vessel walls. We know of no recorded case of amyloid deposition manifesting as a tumor of the testis. Biopsy of the testis is more sensitive than rectal biopsy for the diagnosis of primary and secondary amyloidosis. Most patients with amyloidosis involving the testis also had pathologic changes associated with infertility.
PATHOLOGY OF MALE INFERTILITY
There are several ways to approach the pathology of the testis in the infertile male. Classifications have been oriented toward both morphologic changes and their etiology (84). We use a classification that is principally morphologic but uses known or suspected clinical associations in the case of karyotypic abnormalities and excurrent duct obstruction (Table 46.1).

Biopsy interpretation can be aided by clinical input regarding medical history, including previous paternity, semen analysis, physical findings, and the results of serum gonadotropin measurements. These should not alter the interpretation of the biopsy but, instead, should offer meaningful explanation of the changes and may help with prognostic implications.
NORMAL SPERMATOGENESIS
When the biopsy findings correspond to a fully functioning testis (i.e., insofar as we can tell from the biopsy, all tubules are actively undergoing spermatogenesis) (Fig. 46.1), a diagnosis of normal spermatogenesis is made. The landmark article by Heller and Clermont (85) contains information regarding spermatogenesis and the structure of the normal testis. They point out the mosaic appearance of the testis and demonstrate that not every tubule viewed in cross or longitudinal section has complete spermatogenesis. Sperm counts cannot be precisely correlated with testis biopsy findings, but the number of late spermatids correlates best with sperm counts. Once the diagnosis of normal spermatogenesis is made, an attempt should be made to correlate structure with clinical data. An important determination is whether the patient has excurrent duct obstruction, as discussed in “Excurrent Duct Obstruction.” Histologically, normal spermatogenesis may occur in patients with normal sperm counts and low motility. Ultrastructural evaluation of semen may point out structural abnormalities in spermatozoa of men with normal sperm counts, such as in patients with round-headed sperm or in patients with the immotile-cilia syndrome (86).
HYPOSPERMATOGENESIS
Hypospermatogenesis characterizes a reduced degree of spermatogenesis but not one that stops at a particular point in the sequence of spermatogenesis. It is characterized by appreciable reduction in the number of germinal cells (Fig. 46.11). It may result in a thinning in the number of epithelial layers. Changes in occasional tubules are interpreted as mild, and significant reductions in numbers of germ cells in virtually all tubules are interpreted as severe. Almost invariably, there are other changes, including tunica propria thickening with increased collagen deposition and interstitial fibrosis. Some tubules may show sclerosis, and there may be an appearance of germinal cell disorganization and sloughing into the tubular lumens. Because tubular sclerosis may be only focal within the testis, such changes should not be assumed to be generalized. Sloughing and disorganization may be, at least in part, a function of the technical aspects of obtaining or processing the biopsy. Leydig cells should be present in the angles between the tubules. In general, there is no accurate way to relate their numbers to the level of serum testosterone. There are several methods of evaluating the numbers of Leydig cells in biopsy specimens (2). Only when Leydig cells are absent or virtually absent is their number likely to be associated with a low level of serum testosterone. When Leydig cells appear numerous, particularly when there is coalescence of Leydig cell groups or if nodules are evident, there is probably an increased level of serum LH.
The finding of hypospermatogenesis does not indicate the etiology of the changes. Such changes are not etiologically specific and may occur in a variety of circumstances, including (a) exposure to toxins or excess heat, (b) varicocele, or (c) hypothyroidism.
MATURATION ARREST
The diagnosis of complete maturation arrest is rendered when the germ cells in the seminiferous tubules mature only to a certain point. If spermatogenesis is perceived in a diagrammatic fashion, the appearance suggests that a line exists beyond which germ cell maturation does not extend. Cells with irregular, dense nuclei may be present in the lumens of some tubules and may represent abortive attempts at further maturation or degenerating cells, possibly spermatids (Fig. 46.14A). In incomplete maturation arrest, the appearance is similar, except that a few late spermatids are evident along the luminal border of a few seminiferous tubules (Fig. 46.14B). In both complete and incomplete maturation arrest, tubules are usually reduced in diameter, but there may be no increase in tubule wall thickening or histologic abnormality of interstitium or Leydig cells.
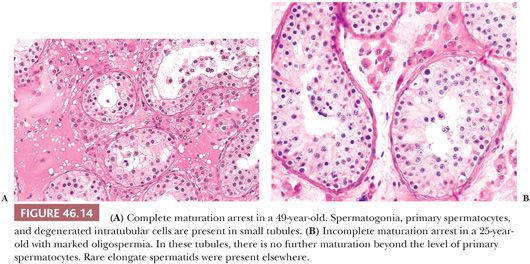
The specific etiology of the changes cannot be predicted from the histology. The same clinical situations that produce hypospermatogenesis may result in germinal cell arrest (84). In addition, postpubertal gonadotropin deficiency, alkylating agent therapy, and radiation therapy may produce similar changes at some stage of injury.
Correlation of structure and sperm count is usually quite good in cases of maturation arrest. In patients with complete maturation arrest, sperm counts are usually zero. When a few sperm are present in ejaculates, the biopsy showing complete maturation arrest may not be representative of both testes, and tubules with late spermatids must be present elsewhere in the testes. When fresh testis biopsies have been minced in patients undergoing assisted reproductive techniques, some testes with a diagnosis of complete maturation arrest have been found to contain spermatozoa. Patients with incomplete maturation arrest are usually oligospermic.
GERM CELL APLASIA
In biopsies of germ cell aplasia (GCA), seminiferous tubules of reduced diameter are lined exclusively by postpubertal Sertoli cells, hence the synonym Sertoli cell–only syndrome (SCO) (Fig. 46.15A). Sertoli cells are oriented perpendicular to the basement membrane. Often, cells in small groups along the length of the tubules are parallel to one another, giving an appearance of palm trees waving in a breeze. The appearance of postpubertal Sertoli cell nuclei is probably best recognized in biopsies of GCA, in which the nuclear indentations and nucleoli can be seen in some cells (Fig. 46.15B). Charcot-Böttcher crystals may sometimes be seen in Sertoli cells as thin, almost imperceptible eosinophilic lines extending in various directions (Fig. 46.2). Nistal et al. (39) described variations in Sertoli cell structure in testes in prepubertal gonadotropin deficiency, cryptorchidism, estrogen treatment, chemotherapy, and del Castillo syndrome. In GCA, Leydig cells are usually normal in appearance but they may appear numerous because of reduced tubular diameter and relative increase in their number. Although the biopsy may indicate SCO, some testicular tissue obtained through testicular sperm extraction (TESE) may contain mature spermatids.
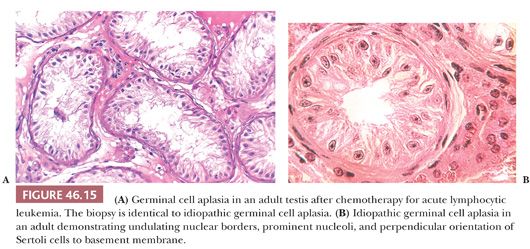
Testes show GCA in del Castillo syndrome, in which phenotypically normal men have small, soft testes, normal secondary sex characteristics, normal levels of serum testosterone, azoospermia, and increased or high-normal levels of serum FSH (87). Occasionally, a biopsy has the appearance of GCA with scattered spermatogonia and is characterized as germinal cell hypoplasia. Biopsies with GCA or germinal cell hypoplasia should be carefully examined to make certain that tubules do not harbor malignant intratubular germ cells. Such cells might be missed if they are few in number and might be misinterpreted if fixation was suboptimal. It must be recognized that alkylating agents and irradiation in sufficient doses over a significant time may produce loss of germ cells and result in the appearance of GCA. In these therapeutic situations, such changes may be reversible over a prolonged period.
GERM CELL APLASIA AND FOCAL SPERMATOGENESIS
Testis biopsies demonstrating GCA with focal spermatogenesis contain two populations of tubules. The smaller tubules exhibit GCA, and tubules of increased diameter show spermatogenesis that is usually reduced (Fig. 46.16). Some tubules, particularly those cut longitudinally, may demonstrate contiguous stretches of only Sertoli cells next to areas with spermatogenesis, indicating that a given tubule may harbor both changes. Patients having bilateral biopsies may show GCA on one side and GCA with focal spermatogenesis or other findings contralaterally. Patients with GCA and focal spermatogenesis usually have a profoundly reduced sperm count.
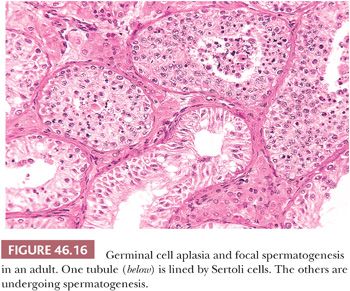
The etiology of GCA with focal spermatogenesis is not apparent from the biopsy. It may be surmised that tubules in some parts of the testis were not populated by germinal cells during intrauterine development, or that postnatally, including childhood and adulthood, there was a loss of germ cells, resulting in tubules with and without germinal cells. If the latter were the case, there might be further loss of germ cells after the biopsy, resulting eventually in complete GCA.
KARYOTYPIC AND CHROMOSOMAL ABNORMALITIES
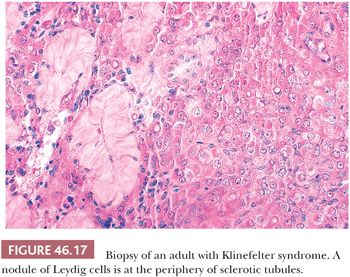
The best known karyotypic abnormality associated with a characteristic appearance of the testis is Klinefelter syndrome (KF) (88), which is characterized by a karyotype of 47,XXY, sometimes a eunuchoid habitus, reduced body and pubic hair, gynecomastia in 40% to 80% of cases, extremely small testes, and an increase of serum FSH and sometimes LH. In KF, the light microscopic structure of the testes may be normal after birth but the number of intratubular germ cells in biopsies of children is reduced (89). Indeed, a significantly reduced number of germ cells have been found in 47,XXY midterm fetuses (90). Some prepubertal testes may contain only Sertoli cells lining tubules. After puberty, the testes usually have a severely altered structure, with reduced spermatogenesis, tubular sclerosis, and Leydig cell nodules (Fig. 46.17). Although some biopsies show absolutely no spermatogenesis, others may show a degree of spermatogenesis. The presence of sperm in the ejaculate does not absolutely exclude the diagnosis of KF. Indeed, Tournaye et al. (91) found spermatozoa in four of nine apparently nonmosaic 47,XXY patients in wet preparations from testicular biopsies. Okada et al. (92) found spermatogenesis in 1 of 8 mosaic and 1 of 140 47,XXY KF patients. The classic histologic appearance of KF may occur in the absence of karyotypic abnormalities. Disease processes in other organs may occur in patients with KF, but only breast carcinoma occurs with increased frequency. Although it has been stated that breast cancer occurs in these patients with the same frequency as in adult females, other studies suggest that this may be an overestimation of the risk (93,94). Rare germ cell tumors have been reported in testes of patients with KF (95). Two cases of epidermoid cyst have been reported in patients with KF (96). At least six intracranial germ cell tumors, mostly seminoma, have been reported in association with KF (97).
Changes identical to KF may occur in patients with a 46,XX karyotype (XX male syndrome). These patients are designated as having de la Chapelle syndrome and are generally shorter than KF patients, with sparse pubic and facial hair; occasional gynecomastia; and possibly reduced size of penis, testes, and prostate (98). These patients may have had an XXY karyotype and may have lost or translocated the Y chromosome early in development. Serum gonadotropin levels are usually increased; serum testosterone level is usually reduced. The fragile X mutation has rarely been found in KF patients with bilateral macro-orchidism (99). Men with infertility and Y chromosome long arm microdeletions may have 45,XO/46,XY chromosome mosaicism (100).
Y chromosome studies have been performed using polymerase chain reaction (PCR). Microdeletions have been identified in region Yq11 that have been subdivided into three subregions: AZFa, AZFb, and AZFc. Microdeletions are associated with a variety of testicular biopsy phenotypes. Brandell et al. (101) identified 9 patients with Y chromosome microdeletions in a group of 80 infertile men (11%). Testicular biopsies showed phenotypes of germinal cell hypoplasia, maturation arrest at spermatid and spermatocyte levels, and hypospermatogenesis. Two patients had discordant phenotypes with GCA in one testis and either hypospermatogenesis or maturation arrest in the other. All patients were azoospermic except one who had a sperm count of less than 1 million/mL. Y chromosome microdeletion does not necessarily indicate infertility. This is probably dependent on the extent and location of microdeletion. Chang et al. (102) reported a Y chromosome microdeletion in a father and four sons. The sons were infertile; one son produced a daughter after intracytoplasmic sperm injection (ICSI). Thus, the Y chromosome microdeletion can be associated with fertility. The deletion can also be passed by ICSI. The presence of Y chromosome microdeletion suggests that an infertile male will not have a stable phenotype in space or in time. Calogero et al. (103) reported a case of repeat biopsies within a year in an azoospermic 25-year-old man with an AZFc microdeletion. His first biopsy demonstrated maturation arrest at a spermatid level. In the second biopsy, there was 90% GCA and 10% maturation arrest at a spermatogonial level, suggesting a continued regression of spermatogenesis (103). Ferlin et al. (104) characterized a 10-year study of Y chromosome microdeletion in an Italian population. Of 3073 consecutive infertile men (625 with nonobstructive azoospermia and 1372 with severe oligospermia), 99 had Y chromosome microdeletion. The overall incidence of microdeletions was 3.2%, with 5% in the azoospermia and oligospermic group. Only 2 of 99 men had a sperm concentration higher than 2 million/mL. The prevalence of cryptorchidism and varicocele was similar in men with and without Yq microdeletion (104).
TUBULAR SCLEROSIS AND INTERSTITIAL FIBROSIS
Although sclerotic seminiferous tubules may be found in biopsies with hypospermatogenesis and frequently are found in cryptorchid testes or those with karyotypic abnormalities, it is rare that biopsies are composed exclusively of sclerotic tubules. This appearance may follow acquired gonadotropin deficiency or remote chronic orchitis or ischemia, or it may occur without a known etiology. Such changes may be accompanied by interstitial fibrosis and loss of Leydig cells and, if present bilaterally and involving the entire testicular parenchyma, can functionally be characterized as end-stage testis.
EXCURRENT DUCT OBSTRUCTION
Obstruction of the excurrent duct system distal to the rete testis occurs in about 10% of biopsied infertile patients. The cause of obstruction may be congenital or acquired. Obstruction may result from agenesis or atresia of part of the excurrent duct system (e.g., the epididymis or vas deferens) or from atrophy resulting from occlusion in cystic fibrosis. Acquired obstruction may result from infection or voluntary sterilization. Occasional inadvertent ligation of the vas deferens may occur during herniorrhaphy or varicocele ligation.
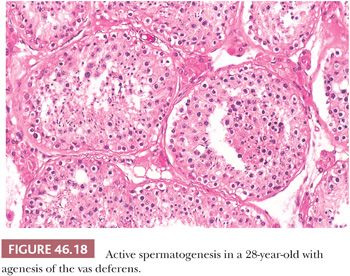
The clinicopathologic triad in patients with excurrent duct obstruction consists of azoospermia, normal-sized testes, and active, although not necessarily normal, spermatogenesis (Fig. 46.18). In a series of nine patients with previous voluntary sterilization 1 to 8 years earlier, we found a variety of changes in biopsies of nine pairs of testes at the time of vasovasostomy (unpublished data) (Table 46.2).
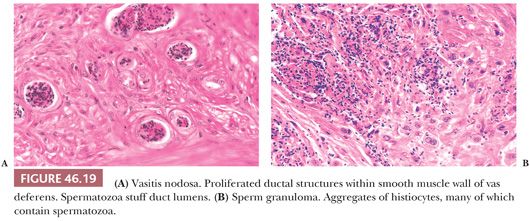
Varying degrees of vasitis nodosa characterized the removed segments of vas deferens. Vasitis nodosa consists of proliferated ductal structures within and through the wall of the vas deferens (105) (Fig. 46.19A). Associated changes are fibrosis, sperm granulomas (Fig. 46.19B), varying degrees of chronic inflammation, elastic tissue proliferation in blood vessel walls, and neural proliferation resembling traumatic neuroma. The ductal structures are lined by cuboidal or columnar epithelium, and some cells are ciliated. Invasion of blood vessels and nerves has been described in vasitis nodosa (106,107). Spermatozoa may be present within some ductal lumens (Fig. 46.19A). Similar lesions were reported rarely without history of prior surgery or trauma (108). The infiltrative pattern may resemble adenocarcinoma, but the history and intraluminal sperm, if present, argue against the diagnosis of malignancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


