Nissen–Rossetti Antireflux Fundoplication (Open Procedure)
David I. Watson
The modern era of antireflux surgery began in the 1950s with the description of fundoplication by Nissen. Nissen’s original procedure entailed a 360-degree wrap of the fundus of the stomach around the distal esophagus. This was constructed using the anterior and posterior walls of the fundus, without dividing the short gastric blood vessels. Subsequently, a number of modifications have been described, with the aim of either simplifying the performance of the procedure, reducing perioperative morbidity, or preventing side effects. In the mid-1960s, Nissen and Rossetti modified Nissen’s original technique so that the anterior wall of the fundus alone was used to construct the 360-degree wrap. Subsequently, DeMeester and Donohue advocated division of the short gastric blood vessels to enable the construction of a “floppy” Nissen fundoplication. More recently, however, the results from four prospective randomized trials of Nissen fundoplication with and without division of the short gastric vessels have demonstrated that an equivalent outcome at follow-up of up to 10 years is achieved without routinely dividing these vessels. Until the early 1990s, the Nissen–Rossetti fundoplication was performed using an open abdominal approach, and, less commonly, thoracic access. Following the development of laparoscopic approaches, a vast majority of primary antireflux procedures are now performed laparoscopically and excellent outcomes have been reported for this approach. This means that the open approach is now used only in a minority of patients undergoing antireflux surgery. Indications for an open surgical approach currently include the following:
Lack of expertise in advanced laparoscopic surgery.
Patient preference.
Completion of a fundoplication that has been converted from a laparoscopic to an open approach (e.g., because of intraoperative difficulties).
Concurrent open surgical procedure.
Reoperative surgery following a previous laparoscopic or open approach.
The first three indications are now quite rare, as laparoscopic approaches are the standard of care for primary antireflux surgery these days. The main indication for open surgery is now a reoperative procedure. Some reoperative surgeries can be completed using a laparoscopic approach. However, a proportion can be performed only via open surgery, and this is more likely following a previous open operation. The transthoracic approach to antireflux surgery has significant morbidity and is now rarely used. It is perhaps best reserved for complex reoperative surgery when at least two previous abdominal operations have been performed—this is rarely undertaken. For this reason, this chapter focuses on the open abdominal approach.
Symptomatic gastroesophageal reflux in patients in whom medical treatment with acid suppression has not adequately relieved symptoms.
Patients with gastroesophageal reflux in whom symptoms are well controlled by medical therapy, but who do not wish to take medication lifelong.
Giant mixed or paraesophageal hiatus hernia where some or all of the symptoms are mechanical (e.g., chest pain, intermittent dysphagia)—that is, not due to reflux alone.
The majority of patients fall into the first category.
It is important to take a complete history and to confirm that the symptoms are actually due to gastroesophageal reflux. Typical reflux symptoms include heartburn, water brash (volume regurgitation of acid-tasting liquid), and epigastric pain. Other symptoms that can be caused by reflux include dysphagia, odynophagia, postprandial fullness, early satiety, upper abdominal bloating, anorexia, nausea, chronic cough, voice change, throat irritation, and wheezing. In general, surgery is very effective for controlling typical symptoms, whereas the response of atypical symptoms to fundoplication is variable.
Endoscopy should be performed in all patients to determine the presence or absence of ulcerative esophagitis, Barrett esophagus, or hiatal hernia. Up to 50% of patients presenting for surgery have no evidence of esophagitis at endoscopy, often because they are using proton pump inhibitor medication. In these patients 24-hour pH monitoring is required to confirm the diagnosis of reflux. Although many surgeons use pH monitoring routinely, the author’s preference is a selective approach in which pH monitoring is used primarily to investigate patients who do not have typical reflux symptoms and evidence of reflux at endoscopy.
Esophageal manometry, however, should be performed in all patients undergoing Nissen–Rossetti fundoplication. It identifies the occasional patient with achalasia, and these patients require different treatment. It also identifies a subgroup of patients who have disordered esophageal body motility, and in these patients a partial fundoplication may be better surgical approach. Barium-meal radiography is not performed routinely, although it is useful to evaluate and document a large hiatal hernia.
Esophageal manometry, however, should be performed in all patients undergoing Nissen–Rossetti fundoplication. It identifies the occasional patient with achalasia, and these patients require different treatment. It also identifies a subgroup of patients who have disordered esophageal body motility, and in these patients a partial fundoplication may be better surgical approach. Barium-meal radiography is not performed routinely, although it is useful to evaluate and document a large hiatal hernia.
Positioning and Instrumentation
For the open transabdominal approach, the patient is placed on the operating table in the supine position. Good exposure is essential, and this is achieved by using a sternal retractor that lifts the sternum and the costal arch forward. An abdominal wall retractor is also used to separate the abdominal wound edges laterally. The author’s preference is to use a sternal retractor that attaches by a chain to a cross bar positioned above the patient’s face. An alternative is the Rochard retractor, which uses two blades to lift and retract the costal margin. Exposure can be improved further by bending the operating table forward 20 to 30 degrees to flex the patient at the waist, and then bend the headpiece backward by a similar amount (see Fig. 1). This shifts the abdominal wound in a cranial direction, and flattens the diaphragm to improve access to the hiatal region. If this is done, the sternal retractor must be attached to the headpiece of the operating table. A fixed retraction system (e.g., Iron Intern or Omnitract) is used to retract the left lobe of the liver. Extra-long surgical instruments are usually needed for the operative procedure, especially when operating on men and obese patients. A renal vein retractor or 1-inch wide Deaver retractor is also useful for displacing the esophagus when undertaking hiatal repair.
An upper midline abdominal incision is used for access. Proximally, this should extend between the xiphisternum and the left costal margin, and distally it finishes just above or just below the umbilicus. The aim of the procedure is to form an effective antireflux valve by constructing a 360-degree wrap around the distal esophagus, using the anterior gastric wall.
Hiatal Dissection
The left lobe of the liver is displaced forward and to the right to expose the upper stomach and the region of the esophageal diaphragmatic hiatus. It is not necessary to divide the triangular ligament of the left lobe of the liver to displace the lobe medially, except on the rare occasion when the liver is excessively enlarged.
The stomach is retracted downward, and the lesser omentum is opened above and below the hepatic branch of the vagus nerve (which is preserved), to display the caudate lobe of the liver and the right hiatal pillar. The phrenoesophageal ligament is divided next (Fig. 2). This comprises two layers, a superficial layer of peritoneum and a deeper fibrous tissue layer. Both layers are divided carefully, so that the layer of tissue that covers both sides of the muscle of the right and left hiatal pillars is preserved. The aim of this initial dissection is to display the edges of both hiatal pillars, as far posteriorly as possible, stopping short of the uppermost short gastric blood vessel on the left side. The anterior and lateral aspects of the lower 4 to 6 cm of the esophagus are displayed during this dissection.
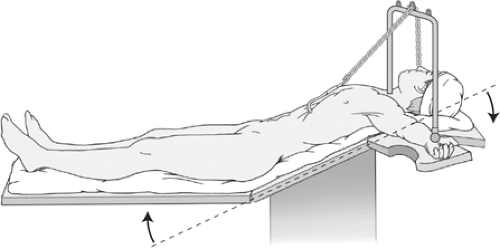 Fig. 1. Positioning for open Nissen–Rossetti fundoplication: overall head-up tilt, hips flexed, sternal retractor, and neck extended. |
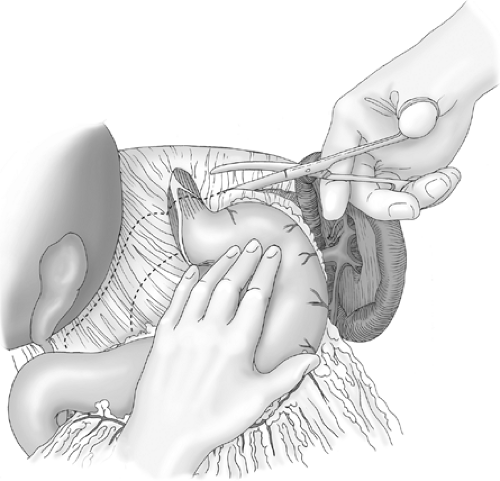 Fig. 2. Division of the phrenoesophageal ligament and opening the lesser omentum above and below the hepatic branch of the vagus nerve. |
Care is taken to preserve both vagus nerves. The anterior vagal branches run along the anterior wall of the esophagus.
They are not formally dissected from the esophageal wall, and hence they are routinely included in the fundoplication. The hepatic branch of the anterior vagus nerve is preserved, and the fundoplication is constructed so that it sits above this. The posterior vagus nerve usually sits separately behind the esophagus. It can be readily identified, and it is usually displaced further posteriorly and excluded from the fundoplication. However, if it is attached to the esophagus by a thick layer of connective tissue, it can be included in the fundoplication.
They are not formally dissected from the esophageal wall, and hence they are routinely included in the fundoplication. The hepatic branch of the anterior vagus nerve is preserved, and the fundoplication is constructed so that it sits above this. The posterior vagus nerve usually sits separately behind the esophagus. It can be readily identified, and it is usually displaced further posteriorly and excluded from the fundoplication. However, if it is attached to the esophagus by a thick layer of connective tissue, it can be included in the fundoplication.
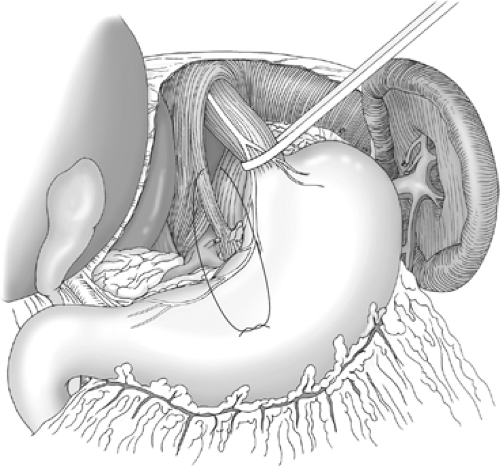
The longitudinal muscle of the distal esophagus is gradually freed from its surroundings by blunt finger dissection, and a space is dissected behind the esophagus, from left to right, using the index and middle fingers. Once a posterior “window” has been created, a nylon tape or a plastic tube is passed around the distal esophagus. Traction is applied to the tape to lift the esophagus anteriorly, and this exposes the posterior hiatus (Fig. 3). The posterior aspect of the distal 6 cm of the esophagus is then fully mobilized. The posterior aspect of the upper part of the stomach can also be separated from the diaphragm at this stage if necessary.
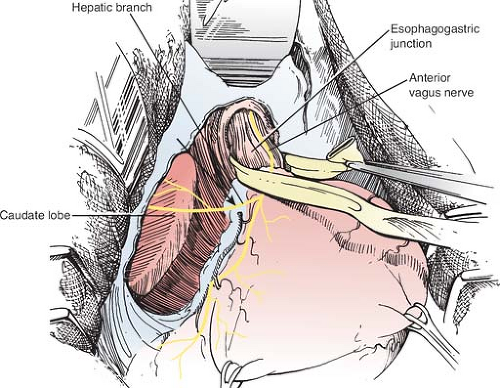 Fig. 3. Tape passed around the esophagus, lifting the esophagogastric junction forward to display the posterior hiatus. |
Mobilization of the esophagus is the most dangerous part of the operation, and there is a small risk of esophageal perforation during this step. This risk is greatest if the esophageal wall is inflamed, edematous, or friable, or if previous hiatal surgery has been performed. The consequences of perforation may be greater if it occurs through the posterior wall, as a transmural lesion in the back wall of the esophagus can easily be overlooked. As long as dissection is in the correct layer, there is little risk of hemorrhage, as the divided structures contain only minute vessels. For this reason, if dissection is performed correctly, it will be relatively bloodless. The surgeon must, however, watch for an accessory left gastric artery running with the hepatic branch of the vagus nerve. If this nerve is preserved, injury to the vessel is unlikely.
Care should also be taken with the left inferior phrenic artery. This runs dorsally to the cardia and fundus, with one or more branches arising directly from the aorta or celiac trunk. It can be, but is not often, damaged during mobilization of the anterior surface of the fundus. In approximately 5% of patients, it arises from the left gastric artery and runs along the edge of the right hiatal pillar. In this situation, it should be ligated and divided to facilitate hiatal dissection. If necessary, the upper short gastric vessels can also be divided, although this is required in less than 5% of Nissen fundoplications.
Hiatal Repair
The hiatus is routinely repaired using posteriorly placed sutures. These should narrow the hiatus to approximately 2.5 cm in diameter (Fig. 4). To do this, the reconstructed hiatal rim should sit loosely (not tightly) around the esophagus with a 52-Fr bougie sited across the gastroesophageal junction. In patients without a hernia or with a small hiatal hernia, one or two interrupted 2-0 nonabsorbable sutures should be placed. If a larger hernia is present, more sutures should be placed. When repairing the hiatus, the right and
left hiatal pillars are exposed posteriorly by retracting the abdominal esophagus to the left with a renal vein retractor or similar narrow-bladed retractor. The first suture is placed through the pillars from left to right, immediately in front of the posterior junction of the two pillars. Subsequent sutures are placed progressively anteriorly to each previous suture, until the hiatal opening is reduced to the appropriate size. If the hernia defect is very large, the posterior sutures can be supplemented if necessary with anterior hiatal sutures, or a piece of prosthetic material can be used to reinforce the repair. If a bougie is used to calibrate the hiatal repair, the repair is performed first without the bougie, and the bougie is passed subsequently to assess the hiatal size.
left hiatal pillars are exposed posteriorly by retracting the abdominal esophagus to the left with a renal vein retractor or similar narrow-bladed retractor. The first suture is placed through the pillars from left to right, immediately in front of the posterior junction of the two pillars. Subsequent sutures are placed progressively anteriorly to each previous suture, until the hiatal opening is reduced to the appropriate size. If the hernia defect is very large, the posterior sutures can be supplemented if necessary with anterior hiatal sutures, or a piece of prosthetic material can be used to reinforce the repair. If a bougie is used to calibrate the hiatal repair, the repair is performed first without the bougie, and the bougie is passed subsequently to assess the hiatal size.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree