Small-Diameter Interposition Shunt
Alexander S. Rosemurgy
Jonathan M. Hernandez
Operative shunting using partial portal decompression gained popularity after its reintroduction by Sarfeh et al. in the early 1980s. Numerous publications and works since then have shown that the concept is safe and efficacious. Sarfeh et al. also studied patients undergoing various degrees of partial portal decompression and found that smaller (8 to 10 mm) prosthetic H-graft portacaval shunts lead to superior results relative to larger (12 to 16 mm) prosthetic H-graft portacaval shunts. Our experience over the past 20 years with partial portal decompression at the University of South Florida confirms earlier work, promoting application of small-diameter (e.g., 8 to 10 mm) prosthetic H-graft portacaval shunts.
Currently, operative shunting for portal hypertension has become limited to a few centers around the United States. This is due to the perceived adequacy of transjugular intrahepatic portosystemic shunts (TIPS) by radiologists and nonsurgeons. TIPS is convenient for the admitting physician given that it can be ordered, not unlike morning labs, thus avoiding surgery consultation and potential operating room delays. With the progressive exclusion of surgeons in the care of patients with portal hypertension, surgeons have generally become less interested in the care of these patients, while the role for nonsurgeons has continuously expanded. For example, the American Association for the Study of Liver Diseases recently published guidelines recommending that “the decision to perform a TIPS, especially in a high-risk patient, should be reached by a team consisting of a gastroenterologist/hepatologist, interventional radiologist, and where appropriate, a transplant physician.” This statement is telling for the perceived need for surgeon expertise.
Hepatic transplantation has further diminished the role of operative shunting in the management of patients with complicated portal hypertension. Hepatic transplantation has become widely available and represents a potentially curative modality for patients with liver disease, including those with complicated portal hypertension, demonstrating 1-year and 5-year patient survival rates of approximately 90% and 80%, respectively. Hepatic transplantation is, however, predicated upon organ availability, which is limited. In addition, transplantation is encumbered by cost considerations and psychosocial requisites that are often prohibitive to potential candidates. As well, it must be recognized that the number of candidates far exceeds the number of organs allocated. Nevertheless, the possibility, not the probability, for hepatic transplantation has served as an impetus for widespread implementation of TIPS given its efficacy as a “bridge” to imminent transplantation. It must be noted that transplantation after TIPS is almost never “imminent.” This bridge is often built but is infrequently crossed, as currently only 10% of patients undergoing TIPS eventually undergo hepatic transplantation.
TIPS should, however, play a role in the management of patients with portal hypertension, irrespective of the possibility for imminent (i.e., within 6 months) transplantation. TIPS should preferentially be undertaken, relative to operatively placed portosystemic shunts, in patients with a history of or current variceal hemorrhage failing or not amenable to endoscopic therapy with concomitant relative contraindications to operative shunting. Relative contraindications to operative shunting include significant congestive heart failure, severe tricuspid regurgitation, severe pulmonary hypertension (mean pulmonary artery pressures >45 mm Hg), severe mitral regurgitation with aortic stenosis, morbid obesity, and/or a hostile abdomen (secondary to significant previous right upper quadrant surgery).
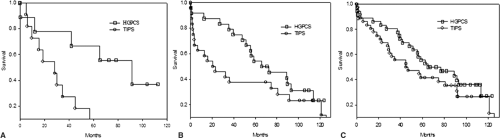
Exclusive of the patient subpopulation mentioned above, we believe sufficient data exist to support preferential application of surgical shunts in general and small-diameter prosthetic H-graft portacaval shunts in particular over TIPS in the management of patients with portal hypertension complicated by variceal bleeding refractory to or not amenable to endoscopic therapy. In particular, we have demonstrated a survival advantage for patients with decent hepatic reserve undergoing small-diameter prosthetic H-graft portacaval shunts relative to patients undergoing TIPS (Fig. 1). The survival advantage, we believe, derives from relative preservation of nutrient hepatic blood flow (i.e., effective hepatic blood flow) for patients undergoing small-diameter prosthetic H-graft portacaval shunts (Table 1). Therefore, to promote survival, appropriate surgical candidates with
adequate hepatic reserve (i.e., Child class A or B or Model for End-Stage Liver Disease [MELD] score less than 13) should undergo small-diameter prosthetic H-graft portacaval shunts. Patients with poor hepatic reserve, defined as Child class C or a MELD score of 13 or more, experience relatively high 30-day mortality rates irrespective of the type of portal decompressive shunt utilized. Although small-diameter prosthetic H-graft portacaval shunts impart no survival advantage relative to TIPS for patients with poor hepatic reserve, they do promote preservation of resources (e.g., blood transfusions, intensive care unit (ICU) days, rehospitalizations) after shunting relative to TIPS and should be preferred unless hepatic transplantation is truly imminent. Notably, hepatic transplantation is generally not undertaken on patients with MELD scores less than 17.
adequate hepatic reserve (i.e., Child class A or B or Model for End-Stage Liver Disease [MELD] score less than 13) should undergo small-diameter prosthetic H-graft portacaval shunts. Patients with poor hepatic reserve, defined as Child class C or a MELD score of 13 or more, experience relatively high 30-day mortality rates irrespective of the type of portal decompressive shunt utilized. Although small-diameter prosthetic H-graft portacaval shunts impart no survival advantage relative to TIPS for patients with poor hepatic reserve, they do promote preservation of resources (e.g., blood transfusions, intensive care unit (ICU) days, rehospitalizations) after shunting relative to TIPS and should be preferred unless hepatic transplantation is truly imminent. Notably, hepatic transplantation is generally not undertaken on patients with MELD scores less than 17.
Table 1 The Impact of Small-Diameter Prosthetic H-Graft Portacaval Shunts and TIPS on Effective Hepatic Blood Flow Measured Utilizing Low-Dose Galactose Clearance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
We have modified original descriptions of prosthetic H-graft portacaval shunting to achieve an approach with which we are satisfied. Relative to others, we use a graft that is shorter, and remove a portion of the caudate lobe to accommodate placement of the shorter graft. As well, we do not aggressively attempt to ligate collaterals from the portal vein. If necessary, occlusion of these collaterals can be completed during a transfemoral shunt study, which we routinely obtain on postoperative day 2 to 4 to document patency.
A diligent second assistant is important to the operation. Such an assistant is necessary to suction accumulating ascitic fluid while the graft–cava anastomosis is undertaken and to keep the graft full of heparinized saline during portal vein–graft anastomosis. The operating surgeon generally stands to the patient’s right side while undertaking this operation, while the first and second assistants stand to the patient’s left.
After the patient is positioned supine on the operating table, an endotracheal tube is placed, and then a Foley catheter and a nasogastric tube are placed. Neither vasopressin nor octreotide is given perioperatively, unless active bleeding is occurring. Although such agents may decrease operative blood loss, we generally have not found blood loss to be significant. The operation can be undertaken without significant blood loss and almost always without blood transfusions. Prophylactic antibiotic therapy is always used.
The patient is rolled into a 30-degree left lateral decubitus position by means of a bed sheet rolled tightly and placed just to the right of the spine. After being prepared and draped in the usual manner, the patient is operated on through a transverse upper abdominal incision. The exact placement of this incision depends on the size of the liver, which is often palpable below the costal margin. We place the incision over the liver edge, and generally do not cross the midline. In making this incision, we usually incise only a small portion of the musculature lateral to the rectus muscle. As is our preference, we use wound protectors and Clorpactin solution for irrigation. Once the peritoneal cavity is entered, we irrigate the wound liberally with this solution and place a wound protector so that the wound is bathed by Clorpactin solution throughout the remainder of the operation. A table-mounted Omni bariatric retractor is used during these operations. All retractor blades are well padded, and the sternal blade of the Omni bariatric retractor is placed in the midportion of the incision to move it cephalad.
If the falciform ligament is divided during the incision, it is divided carefully because large collaterals may be present. Suture ligation of the falciform ligament at the time of its division is usually employed. The foramen of Winslow is a key landmark. A Kocher maneuver is undertaken, always maintaining orientation with the foramen of Winslow. High-energy electrocautery is liberally used, although large visible venous collaterals are ligated before division, as are lymphatic channels. A common mistake in the early part of this operation is that, with the patient in a 30-degree left lateral decubitus position, the dissection is undertaken in a much too lateral direction, deviating from the vena cava. With this error in dissection, the renal capsule or hilum is often exposed needlessly. The foramen of Winslow is a landmark to keep the dissection oriented. The Kocher maneuver does not need to be extensive but just enough to expose approximately 5 cm of inferior vena cava to allow for placement of a side-biting vascular clamp. The exposed segment of the inferior vena cava should include the portion of the inferior vena cava that forms the caudal boundary of the foramen of Winslow. The cephalad portion of this segment of cava may lie dorsal to the inferior tip of the caudate lobe. If necessary, this portion of the caudate lobe is excised to expose the vena cava, so that the portal vein can be bridged to the vena cava easily with a short segment (i.e., 3 cm) of polytetrafluoroethylene (PTFE) graft. To remove a portion of the caudate lobe, the tip of the caudate lobe is grasped using a ringed forceps. Electrocautery is used to divide the caudate lobe from the rest of the liver. Then pressure is placed on the cut edge of the liver to control bleeding. Bleeding veins can generally be controlled with electrocautery, but suture ligation may occasionally be necessary.
It is very important that the inferior vena cava be well exposed medially and laterally so that a vascular clamp can be placed on the inferior vena cava easily. We generally try to expose at least half the circumference of the 4- to 5-cm segment of the inferior vena cava to facilitate clamp placement and anastomosis. After the inferior vena cava is exposed, a traction suture is placed into the loose tissue adjacent to the right side of the inferior vena cava. This suture is further placed into the lateral abdominal wall and then tied to retract this tissue laterally to optimize exposure.
Once the inferior vena cava has been adequately exposed, attention is turned to the hepatoduodenal ligament. With the splanchnic blade of the Omni retractor placed carefully over a well-padded gallbladder, the gallbladder is retracted toward the patient’s left shoulder. This lifts and rolls the gallbladder and the common bile duct ventrally and medially. The hepatoduodenal ligament is opened posteriorly and laterally. This area is rich with lymph vessels, and liberal ligation of lymphatic structures is important in minimizing ascites. The dissection in the hepatoduodenal ligament is undertaken along the length of the ligament, parallel and dorsal to the common bile duct, to minimize chances of ductal injury. Often, the portal vein is not readily identified because of thick lymphatic tissue in this area. Once the portal
vein is identified, further dissection should be undertaken only to better define the portal vein. The common bile duct should be retracted ventrally and medially with a vein retractor to facilitate exposure of the portal vein. Palpation of this area is important to detect and locate an accessory or aberrant right hepatic artery. An accessory or replaced right hepatic artery should not be injured because such an injury can greatly interfere with necessary blood flow to the liver. If present, an aberrant or accessory right hepatic artery should be retracted with the common bile duct and thereby retracted ventrally and medially. As the portal vein comes into view, a Russian forceps is used to grasp the portal vein. A substantial portion of vein should always be grasped to minimize chances of tearing it. Dissection of the portal vein is greatly facilitated with use of a plastic Yankauer sucker (Fig. 2).
vein is identified, further dissection should be undertaken only to better define the portal vein. The common bile duct should be retracted ventrally and medially with a vein retractor to facilitate exposure of the portal vein. Palpation of this area is important to detect and locate an accessory or aberrant right hepatic artery. An accessory or replaced right hepatic artery should not be injured because such an injury can greatly interfere with necessary blood flow to the liver. If present, an aberrant or accessory right hepatic artery should be retracted with the common bile duct and thereby retracted ventrally and medially. As the portal vein comes into view, a Russian forceps is used to grasp the portal vein. A substantial portion of vein should always be grasped to minimize chances of tearing it. Dissection of the portal vein is greatly facilitated with use of a plastic Yankauer sucker (Fig. 2).
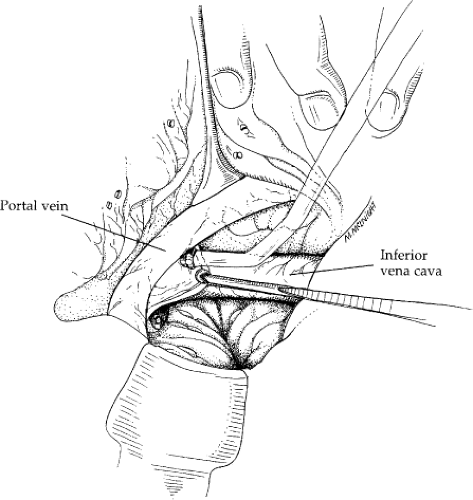 Fig. 2. The Yankauer sucker is used to raise the portal vein off the tissue of the posterior hepatoduodenal ligament. A Russian forceps is used to minimize chances of tearing tissue. A vein retractor is helpful in the dissection. Here, it is retracting tissue containing the common bile duct medially. The vena cava is seen at the base of this view. A portion of the caudate lobe has already been excised. The line of excision of the caudate lobe lies posterior to the cephalad aspect of the portal vein. The patient’s head is to the left. This view is from the patient’s right side, where the surgeon is standing.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|