Comprehensive Parathyroidectomy for the Treatment of PHPT
Allan E. Siperstein
Mira Milas
Introduction
The modern era of parathyroid disease management is characterized by three fundamentally new and important features. First is the evolution of primary hyperparathyroidism (PHPT) from a rare endocrine disorder to the most common cause of hypercalcemia in the outpatient population, with estimated prevalence of 1 in 500 women and 1 in 2,000 men. A second and related feature is higher prevalence of asymptomatic PHPT, where the clear biochemical diagnosis of parathyroid disease exists without noticeable symptoms or clinically detectable consequences, such as bone density loss or kidney stones. More patients are being diagnosed in such an asymptomatic phase of the disease because calcium has become a routine component of automated chemistry panels, thus leading to incidental detection of hypercalcemia. Conversely, more practitioners are also recognizing the need to screen patients with osteoporosis, osteopenia, and kidney stones for underlying PHPT utilizing not just calcium, but a panel that includes calcium, intact parathyroid hormone (PTH), and 25-hydroxyvitamin D levels.
In response to these evolving presentations of parathyroid disease, several multidisciplinary publications have offered guidelines for the indications and timing of parathyroid surgery. Despite these guidelines, patients with PHPT remain, as a group, underreferred and undertreated surgically. Surgical therapy remains the only definite and durable treatment for PHPT. What was once designated “conventional,” “traditional,” or “bilateral” parathyroidectomy, however, may not be the most suitable terminology for present-day and future surgeons. The third important feature of current surgical management of parathyroid disease is, therefore, that there has been a clear paradigm shift toward focused parathyroid surgery: the exploration of a single site of suspected parathyroid gland abnormality. Most parathyroid surgeons have adapted this as the favored initial approach to parathyroid surgery, guided by radiologic studies and intraoperative PTH measurement.
This chapter, however, is devoted to the approach of a comprehensive parathyroidectomy, a term we have designated to mean examination of all parathyroid glands bilaterally with appropriate resection of diseased glands. It is imperative to recognize that “conventional” or “bilateral” parathyroid procedures are not obsolete but in fact remain essential. Comprehensive parathyroidectomy will remain integral to the surgical treatment of PHPT and, for appropriate patients, is the ideal initial operation. Comprehensive parathyroid examination can be performed in a minimally invasive way. This approach requires a thorough understanding of parathyroid gland anatomy and embryology and of specific indications based on clinical presentation, parathyroid imaging, and intraoperative findings. In this chapter, we review the key roles of comprehensive parathyroidectomy as part of the spectrum of available parathyroid operations.
There are typically four parathyroid glands in most individuals. A large autopsy study identified four parathyroid glands in 84% of human cadavers, five or more glands in 13%, and only three parathyroids in 3%. Supranumerary parathyroids are most often located in the thymus. The possibility of having an unusual number or location of parathyroid glands has direct impact on the success of parathyroid surgery and the potential need for comprehensive parathyroidectomy.
A brief survey of the history of parathyroid surgery illustrates the importance of applying the knowledge of parathyroid anatomy and embryology during parathyroidectomy. Normal parathyroid glands are approximately 5 to 6 mm in greatest dimension, weigh 15 to 35 mg, and can be inconspicuous with their orangetan color embedded or flattened within a surrounding yellow fatty tissue envelope. Thus, they were only first identified in a large mammal, the Indian Rhinoceros. Following this discovery by Sir Richard Owen during an autopsy of the rhinoceros in the London Zoo in 1852 and publication as a minor comment about parathyroids in 1862, it was Swedish medical student Ivar Viktor Sandstrom who first identified parathyroids in man in 1875. In the early 1900s, biochemical measurement of calcium became possible and the association between parathyroids and severe bone disease was noted, although the original misconception was that osteitis fibrosa cystica caused parathyroid problems, rather than the reverse. Felix Mandl performed the first successful parathyroidectomy in 1925 in Vienna. Oliver Cope and his colleagues at the Massachusetts General Hospital then contributed significantly to the knowledge of parathyroid anatomical distribution and challenges of ectopic and mediastinal parathyroids. Exemplifying this is the well-known case history of their patient, Captain Charles Martell, who underwent his seventh parathyroid exploration in 1932. From that time period until about 2003, bilateral, comprehensive parathyroid exploration was the dominant surgical approach for the treatment of parathyroid disease.
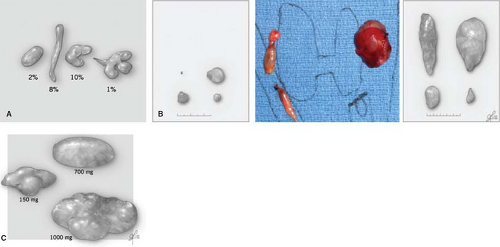
The key aspects of parathyroid anatomy and embryology to adapt to comprehensive parathyroidectomy are illustrated in Figs. 1 to 3. The appearance of parathyroids can be variable even when they are biochemically functioning normally. When diseased, parathyroid glands may display variable morphological changes in size, shape, texture, and firmness. Abnormal parathyroids are generally fuller in all dimensions, have a darker brown or reddish-brown color, and do not compress easily or are significantly firm when gently probed. They may have an irregular and knobby shape, more prominent vascular pedicles, or a plexus of vasculature. Glands of patients with secondary and tertiary hyperparathyroidism may be sclerotic and light in color from this fibrosis. In cases of borderline abnormal appearance, it is helpful to determine in vivo parathyroid weight prior to excision of the parathyroid. This can be readily done by measuring parathyroid length (L), width (W), and height (H) using a small ruler or micrometer device without removing the gland. Since most glands are oval, calculating the volume of an ellipsoid using sizes in millimeters estimates parathyroid gland weight in milligrams (weight [mg] ≈ L × W × H × ½) [mm3]). There is ongoing interest and some controversy in defining what truly constitutes an abnormal parathyroid, and whether this is a matter of purely morphological form, biochemical function, or a combination of both.
Embryologically, the upper parathyroids develop from the fourth branchial pouch and migrate caudally with the thyroid, while the lower parathyroid glands derive from the third branchial pouch and migrate with the thymus. The upper parathyroid glands have a narrow area of distribution and are fairly reliably positioned in the perithyroidal fat posterior to the superior pole of the thyroid gland and near the path of the recurrent laryngeal nerve as it enters the cricothyroid muscle. In contrast, the lower parathyroids are more widespread around the lower pole of the thyroid gland, thyrothymic ligament, and pretracheal fat. Symmetry is usually present between parathyroid locations in the left and right sides of the neck. Additional clues for parathyroid location can come from observing the patterns of vasculature in and around the expected parathyroid region. Both parathyroids typically derive some blood supply from the inferior thyroid artery. In relation to the path of the main trunk of this artery as it nears the thyroid, upper parathyroids are cranial and deeper, and lower parathyroids are caudal, anterior, and medial. Unusually curved or extra branching patterns of the artery may alert to abnormal parathyroids found hanging at the ends of those branches, sometimes several centimeters away from the thyroid capsule. Within their fatty envelope, a normal parathyroid will have a leaf-like branching pattern of their vascular pedicle. This is a helpful contrast to lymph nodes, fat or thymic tissues that have no visible vascular pattern, and abnormal parathyroids whose vascular pedicle may be exaggerated.
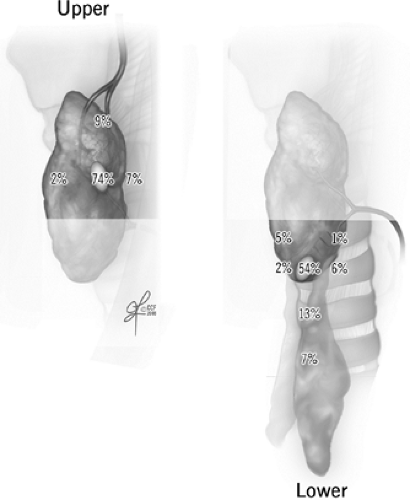 Fig. 2. Normally expected distribution of upper and lower parathyroid glands (see Ritter and Milas in Suggested Readings for additional references). |
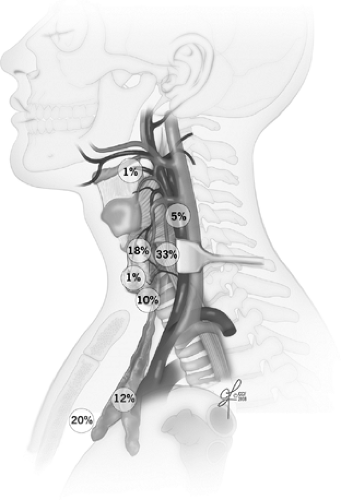
Migratory distribution of the parathyroids can lead to ectopic locations within the thymus, within the sheath encompassing the carotid artery, jugular vein, and vagus nerve even in high cervical locations, retroesophageally and even intrathyroidally. Some, but not all of these, areas can be accessed via the usual cervical incision during comprehensive parathyroid exploration. Recently, novel nomenclature was proposed to further classify cervical parathyroid adenomas into regions relevant for parathyroid exploration (see Suggested Readings). Ectopic parathyroid locations in the anterior mediastinum, other deeper regions of
the mediastinum, and even pericardium require alternate surgical approaches often in collaboration with thoracic surgeons.
the mediastinum, and even pericardium require alternate surgical approaches often in collaboration with thoracic surgeons.
PHPT is a disorder of excessive PTH secretion, derived from single (70% to 90%) or multiple (10% to 30%) benign parathyroid tumors in the vast majority of patients and only rarely (<1%) from parathyroid carcinoma. Only 3% of patients with PHPT have this in the context of multiple endocrine neoplasia (MEN) syndromes.
The excess PTH secretion disbalances multiple aspects of calcium homeostasis, ultimately leading to hypercalcemia. A principal action of PTH targets the kidney to retain calcium and excrete phosphorus and bicarbonate, thereby elevating serum calcium and reducing serum phosphate levels and pH, and causing hypercalciurea and nephrocalcinosis. Chronically high PTH increases osteoclast activity, particularly in cancellous bones, thereby contributing to bone density loss. The effects of PHPT are especially apparent in the distal radius, thus DXA scans measuring osteopenia and osteoporosis should include this region in addition to the usual lumbar spine, hip, and femur sites. PTH also increases gastrointestinal calcium absorption. It upregulates renal hydroxylation of 25-hydroxyvitamin D, and can thus lead to a serum profile of low 25-hydroxyvitamin D and elevated 1,25-dihydroxyvitamin D in some patients. It is intuitive from these physiologic derangements how the clinical presentation of PHPT can include any or all of the following: kidney stones; osteopenia, osteoporosis, and bone fractures; diagnosis of vitamin D deficiency; increased urination and thirst; and vague abdominal aches and constipation. Less clear is the underlying mechanism for the spectrum of additional clinical findings in patients with PHPT: neurocognitive changes such as depression, poor mentation, inability to focus, and insomnia; musculoskeletal aches and weakness; profound fatigue; and rare presentation of pancreatitis. Osteitis fibrosa cystica and Brown tumors are almost historical clinical findings, rarely encountered in such severity at the present time. In contrast, incidental diagnosis at an asymptomatic stage of PHPT is becoming increasingly prevalent, although many reveal subtle symptoms on closer interrogation. The vast majority of patients with PHPT are indeed asymptomatic, most often with kidney stones and bone density loss.
Traditionally, the diagnosis of PHPT has rested on the demonstration of simultaneously elevated serum total and/or ionized calcium with elevated intact PTH, in the setting of normal or high calcium excretion in the urine. With this combination of findings, the diagnosis of PHPT is practically definitive. In part, this is because modern measurements of PTH detect the intact molecule, reflecting the entire protein derived from the parathyroid glands, and essentially eliminating confounding diagnoses from ectopic sources of PTH, such as tumors producing PTH-related peptide (PTHrp). The rare hereditary condition of benign familial hypercalcemic hypocalciurea (BFHH) is excluded by the finding of normal or high levels of calcium in a 24-hour urine collection.
Approximately 10% of patients will have unusual biochemical presentations that do not fit these classical diagnostic criteria, but are nonetheless found to have PHPT. There are at least two atypical versions of the disease. Normocalcemic PHPT manifests with normal total serum calcium but high PTH and has been relatively well appreciated; despite borderline laboratory values, these patients suffer from kidney stones, osteoporosis, and bone fractures. The other form of PHPT has high calcium levels but normal PTH. Diagnosis is somewhat easier if PTH values are “inappropriately” high-normal for the degree of hypercalcemia (40 to 60 pg/mL on a scale where 60 pg/mL is maximal reference range), but can be challenging when values are as low as 15 pg/mL. This atypical version has not been well characterized.
Table 1 provides a recommended diagnostic work-up for PHPT and strategies to clarify the diagnosis in challenging scenarios. It is advisable to obtain a baseline bone density assessment with DXA scan, especially if this did not precede referral of the patient to the surgeon. Urinary calcium excretion lower than 50 mg/dL should prompt consideration of explanations that include BFHH, renal disease, and use of thiazide diuretics, among others.
Although there can be other non-endocrine causes of hypercalcemia coexisting with PHPT, these are exceedingly rare. Separate investigation for these is not warranted at the outset in a patient with elevated calcium and intact PTH whose medical history does not have pertinent findings, such as hypercalcemia-associated malignancies. Such investigation may be helpful in atypical presentations. Obtaining a thorough family history is important
to discern possible MEN and, if suspected, appropriate additional evaluation can be tailored. Routine genetic testing for MEN1 (where 90% manifest parathyroid disease) and MEN2 (where parathyroid disease affects, 5% patients) is unwarranted as part of initial diagnostic work-up for PHPT.
to discern possible MEN and, if suspected, appropriate additional evaluation can be tailored. Routine genetic testing for MEN1 (where 90% manifest parathyroid disease) and MEN2 (where parathyroid disease affects, 5% patients) is unwarranted as part of initial diagnostic work-up for PHPT.
Table 1 Diagnostic Work-Up for Primary Hyperparathyroidism | |||||
|---|---|---|---|---|---|
|
The operative goals for the treatment of proven PHPT are the following:
Achieve a normocalcemic state and normal long-term PTH.
Avoid injury to the laryngeal nerves.
Engender minimal postoperative morbidity and negligible mortality.
Achieve cosmetic scar appearance acceptable to the patient.
Surgery remains the most clearly demonstrated mechanism for durable cure of PHPT and symptomatic improvement, particularly related to osteoporosis and bone fractures, and neurocognitive issues. A number of surgical techniques have evolved over the last decade. These include focal and unilateral exploration guided by intraoperative PTH measurement, radioguided parathyroid surgery, and videoscopic and robotically assisted parathyroidectomy. They all aim to achieve the above goals.
Some patients with PHPT may not be suitable candidates for surgery or have other reasons to forego parathyroidectomy. Percutaneous ethanol ablation, bisphosphonates, and calcimimetic agents have been described as nonoperative treatment options. These medications reduce calcium and PTH levels while administered, but their long-term impact on improving systemic consequences of hyperparathyroidism is unclear. Frequent monitoring of changes in laboratory values, recommendations to avoid dehydration and excess calcium intake, and periodic reassessment for surgery are important components of nonoperative management.
A joint statement in 2005 by national professional associations of endocrine surgeons and endocrinologists stated that operative management is clearly indicated for all patients with classic symptoms or complications of PHPT. More challenging has been the perspective of decision making for those with apparently asymptomatic PHPT. Experts organized by the National Institutes of Health in 2002 proposed parathyroidectomy for the following patients: (a) those <50 years of age, (b) who cannot participate in appropriate follow-up, (c) with a serum calcium level >1.0 mg/dL above the normal range, (d) with urinary calcium >400 mg/24 h, (e) with a 30% decrease in renal function, or (f) with systemic complications of PHPT including nephrocalcinosis, osteoporosis (T-score lower than -2.5 SD at the lumbar spine, hip, or wrist), or a severe psychoneurologic disorder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree