Chapter 19
Neurobiology of Schizophrenia, Mood Disorders, and Anxiety Disorders∗
Schizophrenia
Etiology and Pathophysiology
Genetic Predisposition
Nonetheless, schizophrenia is not a simple genetic disorder in which inherited disease alleles will always lead to illness. Schizophrenia likely involves several genes located on different chromosomes and differs from mendelian disorders, in which genes are fully penetrant and recognized as the primary cause of disease (e.g., genes for Huntington disease). As indicated by the 50% concordance rate in monozygotic twins, the genes for schizophrenia show reduced penetrance, resulting in individuals who carry the disease genes without manifesting the illness. Further complicating the search for the specific genes that confer risk of schizophrenia is the variability in biologic and phenotypic traits among individuals who manifest the illness (see What’s New? Copy Number Variations Increase in Schizophrenia and in Offspring of Older Fathers).
Prenatal and Perinatal Vulnerability Factors
Because the concordance rate of schizophrenia in monozygotic twins is never 100% as in mendelian disorders, environmental factors likely play an important role in increasing the risk of developing the disorder. A leading hypothesis suggests that early environmental factors interfere with genetically programmed neural developmental alterations that eventually compromise normal brain structures and functions.1 An early brain defect may remain silent and not dramatically affect the individual until subsequent development requires adaptive use of brain structures.2 Several hypothesized early environmental factors that may alter brain development and increase the risk of developing schizophrenia include exposure to prenatal infection, prenatal nutritional deficiencies, perinatal complications (such as birth defects and neonatal hypoxia), and upbringing in an urban environment.3
Neuroanatomic and Functional Abnormalities
Neuroanatomic Alterations
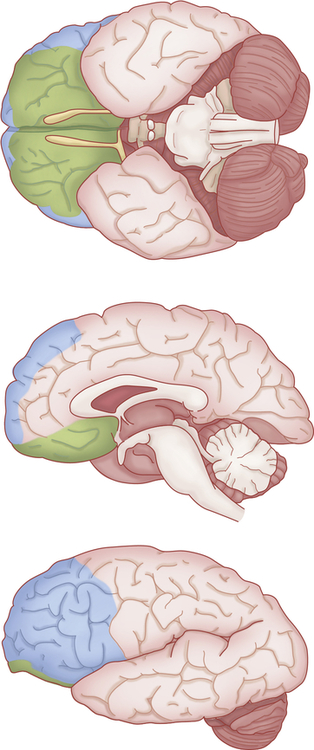
Advanced neuroimaging techniques have revealed evidence of structural brain abnormalities in schizophrenia.4,5 A consistent finding is the enlargement of the lateral and third ventricles and the widening of frontocortical fissures and sulci (Figure 19-1). Schizophrenics with cerebral ventricular enlargement often exhibit cognitive impairments and negative symptoms, and respond poorly to treatment. Other studies reported a reduction in the thalamus and temporal lobe areas (e.g., amygdala, hippocampus, and parahippocampal gyrus).6 A reduction in thalamus size may disrupt neurotransmission between the cortex and primary sensory and motor areas. Temporal lobe alterations may contribute to the production of positive schizophrenic symptoms, such as hallucinations, delusions, and thought disorder.
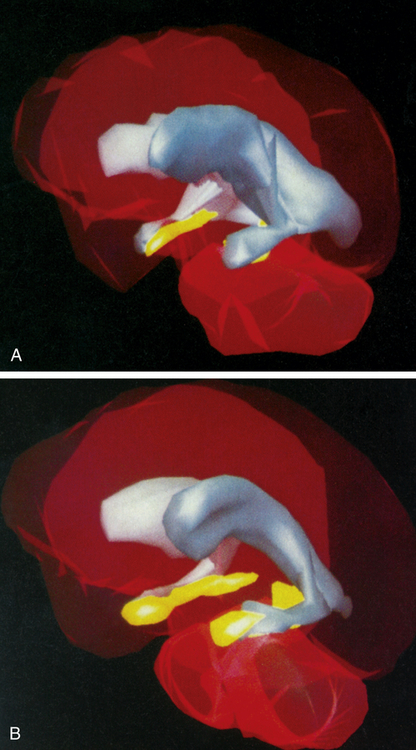
Three-dimensional MRI reconstructions showing A, the cerebroventricles (gray regions) and hippocampus (yellow regions) of a schizophrenic patient, and B, those of a healthy individual. Note the enlarged cerebroventricles and reduced hippocampal volume of the brain of the schizophrenic individual. (From Gershon ES, Rieder RO: Sci Am 267:128, 1992. Original illustrations by Nancy C. Andreason, University of Iowa.)
Brain imaging studies in adolescents with early onset schizophrenia reveal progressive loss of cortical gray matter in temporal lobes, somatosensory and motor cortices, and the dorsolateral cortex (Figure 19-2). Of clinical concern is the loss of cortical tissue, which is evident by the time the individual seeks treatment and continues throughout the course of the illness despite the use of antipsychotic medication.7 The progressive loss in frontal lobe volume is accompanied by increased severity of negative symptoms and further reductions in cognitive functioning. These results highlight the ineffectiveness of current medications for schizophrenia to attenuate or reverse the loss of frontal brain tissue.
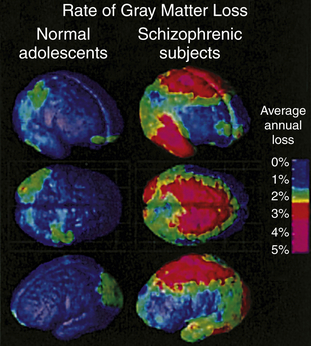
Annual gray matter loss ranging from 2% to 5% was found in 13- to 18-year-old schizophrenics—compared with age-matched healthy adolescents. (From Thompson PM et al: Proc Natl Acad Sci U S A 98:11650, 2001.)
Brain abnormalities in schizophrenia are believed to originate in the prenatal period of cell proliferation and migration. Reelin, an extracellular matrix protein involved in neuronal migration during development and in synaptic function during adulthood, is reduced in the prefrontal cortex and hippocampus of schizophrenic individuals.8,9 Reelin is concentrated in interneurons that contain gamma-aminobutyric acid (GABA), the most widespread inhibitory neurotransmitter. Furthermore, in the dorsal prefrontal cortex of schizophrenic brains, the level of glutamic acid decarboxylase, the major enzyme in GABA biosynthesis, is reduced, which likely impairs normal cognitive/emotional functions.
Pathophysiologic changes in the dorsal prefrontal cortex are believed to contribute to the production of negative symptoms in schizophrenia (Figure 19-3). In particular, the dorsolateral prefrontal cortex (DLPFC) (Brodmann areas 9, 10, 46, 47) is intricately involved in the initiation and maintenance of goal-directed activities and in solving cognitive problems related to working memory. Working memory involves the brief storage and use of information to complete cognitive tasks such as language comprehension, learning, and reasoning. Blood flow and metabolism normally increase in the DLPFC during working memory processing but not in schizophrenics, who also perform poorly in tests of working memory. Thus the dorsolateral prefrontal cortex appears to be hypoactive in schizophrenia.
Neurotransmitter Alterations
A current view of the dopamine hypothesis of schizophrenia is that brain dopamine pathways are altered in different ways (Figure 19-4). For example, the negative symptoms and cognitive alterations in schizophrenia are proposed to result from reduced dopaminergic neurotransmission in the mesocortical dopamine pathway.10 This hypodopaminergic transmission in the prefrontal cortex contrasts with the hypothesized hyperdopaminergic secretion, in mesolimbic brain regions, which may contribute to the production of positive schizophrenic symptoms. The mesolimbic dopamine pathway innervates temporal lobe structures including the hippocampal formation and amygdala, as well as the nucleus accumbens and anterior cingulate cortex.
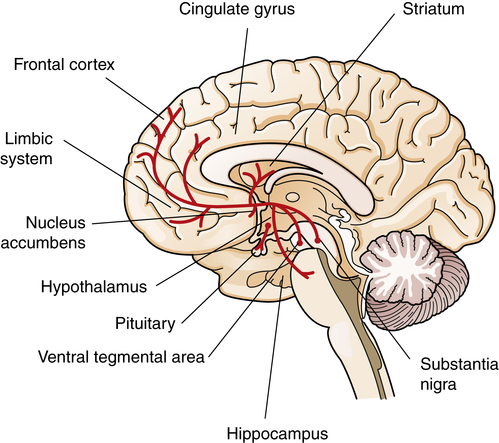
Dopamine cell bodies are located in the substantia nigra, where they project to the stratum (nigrostriatal pathway); and in the ventral tegmental area, where they project to the frontal and cingulate cortex (mesocortical pathway), the striatum, the hippocampus, and other limbic structures (mesolimbic pathway). Dopamine nuclei are also located in the hypothalamus and project to the pituitary.
Another neurotransmitter system that may underlie the pathogenesis of schizophrenia is the excitatory neurotransmitter glutamate and its actions on the N-methyl-d-aspartate (NMDA) receptor subtype. The glutamate hypothesis of schizophrenia proposes that underactivation of glutamate receptors contributes to schizophrenia.11 In schizophrenia, glutamate concentrations in the cerebrospinal fluid (CSF) are reduced along with a decrease in cortical glutamate synthesis. Furthermore, in unaffected individuals, blocking the glutamate NMDA receptor with antagonists, such as phencyclidine (PCP) and ketamine, facilitates the positive and negative symptoms of schizophrenia. PCP users report auditory hallucinations and disorientation, and may become violent from their delusions. In monkeys, chronic PCP treatment impairs cognitive performance in a test associated with prefrontal cortical damage.12
Clinical Manifestations
The symptoms of schizophrenia are currently divided into three broad categories of positive, negative, and cognitive symptoms (Box 19-1). Positive symptoms frequently occur during a psychotic episode, when an individual loses touch with reality and experiences something that should be absent (e.g., hallucinations). Negative symptoms are characterized by disruptions in normal emotional states and expressions. Cognitive symptoms are fairly common and involve problems with thought processes that severely impair the ability to perform routine daily tasks that involve attention, planning, and social skills. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR),13 schizophrenia is diagnosed when an individual exhibits delusions, hallucinations, negative symptoms, or social/occupational dysfunctions for at least 6 months and at least two of the common symptoms of the disorder—delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, or presence of negative symptoms—must occur for 1 month (or less if successfully treated).
Treatment
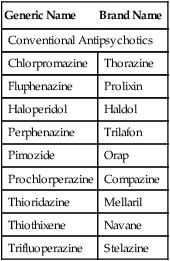
Although the majority of schizophrenic individuals obtained some positive symptom relief from the first-generation or conventional antipsychotics, approximately 20% failed to respond to D2-blocking drugs (Box 19-2), especially those with pronounced symptoms of apathy, disorientation, and social withdrawal. However, some of these treatment-resistant individuals responded to a second generation of drugs that became known as atypical antipsychotic drugs.14 Atypical antipsychotics also were shown to have superior efficacy in reducing not only the positive but also the negative symptoms in comparison with conventional antipsychotics. For example, clozapine improve some cognitive functions (such as verbal fluency, verbal learning, and memory) and some physical functions (such as psychomotor speed). In addition, the notable neurologic side effects that accompany the use of the conventional antipsychotics were diminished.
In conjunction with antipsychotic medication, psychosocial therapy can facilitate the management of schizophrenia. Psychosocial relationships assist the individual in developing coping strategies and in identifying stressors and relapse symptoms. Cognitive-behavioral therapy (CBT), a talking therapy that initiates cognitive and behavioral change based on an individualized reappraisal of the person’s faulty beliefs, is effective in treating schizophrenics with stabilized antipsychotic medications.15 An important benefit of psychosocial and family support is the encouragement of compliance with antipsychotic medication that requires a period before the emergence of clinical efficacy.
Mood Disorders: Depression and Bipolar Disorder
Mood refers to a sustained emotional state as opposed to brief emotional feelings, which are termed affective states. Healthy individuals are normally capable of experiencing a variety of affective states including euphoria, joy, surprise, fear, sadness, anxiety, and depression. When emotional states, such as sadness, become chronic and uncontrollable, individuals may be diagnosed with a mood disorder called depression. The two major classifications of mood disorder are: (1) unipolar or major depressive disorder, also known as major depression or clinical depression, which consists of episodes of depression; and (2) bipolar disorder, which is further classified into bipolar I and bipolar II disorders. Bipolar I disorder features manic episodes and at least one major depressive episode and bipolar II disorder is characterized by recurrent major depressive episodes with one or more hypomanic (milder than manic) episodes. Box 19-3 presents the major criteria of depression and bipolar disorder according to the American Psychiatric Association’s DSM-IV-TR.13