102
CHAPTER OUTLINE
■ DEVELOPMENT OF THE ADOLESCENT BRAIN
■ DEVELOPMENT OF GOAL-DIRECTED BEHAVIOR
■ EVIDENCE FROM NEUROIMAGING STUDIES OF HUMAN DEVELOPMENT
■ BIOLOGIC PREDISPOSITIONS, DEVELOPMENT, AND RISK
■ NEW EVIDENCE OF THE LINK BETWEEN OBESITY AND DRUG ADDICTION
■ ROLE OF FAMILY AND PEERS DURING KEY DEVELOPMENTAL STAGES
Addiction, for the purposes of this chapter, will be defined along the DSM-5 criteria of substance use disorder. The authors will attempt to delineate genetic, biologic, pharmacologic, and social factors that may lead to addiction from a developmental perspective. What factors lead to early experimentation? Is it genes that make youth more susceptible to use, or environment, or both? To answer this, one must understand the role of genes and environment, epigenetics, on the developing brain. In order to illustrate these factors, a case presentation is first discussed; then, the author will begin to explore the biopsychosocial components of this case presentation that increased the risk of developing a substance abuse disorder (SUD) in the case described. As well, the reader is invited to refer to Chapter 1 as background prior to reading this chapter.
BIOPSYCHOSOCIAL ANALYSIS
Constructing a chronologic summary of the biopsychosocial information can help assimilate the important events. This can be done as shown in Figure 102-1.
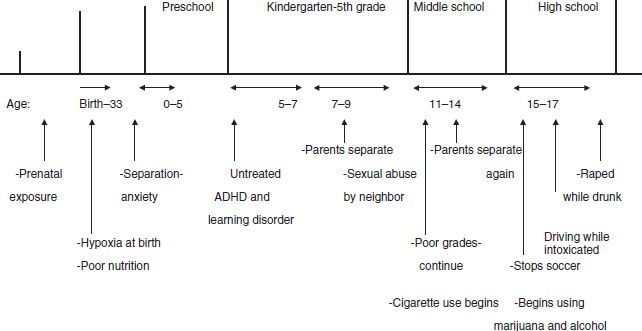
FIGURE 102-1 Developmental trajectory of Linda.
The impact of each of these events as risk factors for substance abuse is reviewed from a developmental perspective in the next section. When appropriate, the implication of these events on the developing brain from a neurobiologic perspective will be explored in the chapter.
Prenatal Exposure
Although it is unclear whether prenatal exposure to substances of abuse can increase the risk of developing an SUD, studies have shown that prenatal exposure can increase the risk of developing a learning disorder (LD) or comorbid conditions that may increase the risk of abusing drugs and alcohol. In this case study, the fetus was subjected to cigarette and marijuana smoking.
Prenatal Exposure to Nicotine and Marijuana
Prenatal nicotine exposure can increase a toddler’s negativity and their propensity for externalizing disorders, such as ADHD or ADHD symptoms and/or conduct disorders (1,2). Prenatal cigarette exposure has also been associated with lower IQ, poorer auditory functioning, and poorer performance on tests requiring fundamental aspects of visual– perceptual performance. In contrast, prenatal marijuana exposure did not have a negative impact on IQ, auditory-based behaviors, or basic visual–perceptual skills. Rather, in utero exposure to marijuana impacted on the application of these skills in problem-solving situations requiring visual integration and analytical skills, as well as sustained attention. A study involving multiple regression analyses (including controls with no exposure) in 18- to 22-year-olds exposed prenatally to marijuana confirmed the effect on visual–spatial working memory (3).
Linda’s history of early colicky behavior and possible LDs and reading difficulty may have been partly precipitated by early nicotine exposure. However, the fetus was also exposed to marijuana. Consistent patterns of cognitive deficits related to prenatal exposure to marijuana have been found from a longitudinal study (4). The cohort of women included women ranged in age from 18 to 42; half were African American and half were white, and most were of lower socioeconomic status. These include IQ deficits and inattention at age 3. At age 6, the history of exposure to heavy marijuana maternal use (one or more cigarettes per day) during the first trimester was associated with lower verbal reasoning scores on the Stanford-Binet Intelligence Scale. Exposure to heavy use during the second trimester predicted deficits in the composite, short-term memory, and quantitative scores on the Stanford-Binet (5). At 6 and 10 years of age, offspring were more impulsive and had more internalizing and externalizing behaviors. At 14 years of age, prenatal marijuana predicted executive function difficulty, attention deficit problems, and an increased risk for substance use. At 16 years of age, externalizing behaviors, psychiatric disorders, and substance abuse were associated with prenatal marijuana exposure. All analyses controlled for significant covariates (3). Though Linda’s mother stopped using in the first trimester, the use during this time period may have contributed to problems with Linda’s reading ability.
CASE PRESENTATION
Linda, a 17-year-old female high school student, presents to your office. She is accompanied by her parents, who are worried about drug use. Linda was pulled over by the police after a party for reckless driving. She was found to have an alcohol level of 0.25 and was charged with a driving under the influence. She was ordered by the court to be evaluated and enter a drug treatment program.
Developmental History
Mom revealed that, prior to her pregnancy with Linda, she smoked a pack of cigarettes a day and had a glass of wine once a week. She also admitted to using marijuana daily. Upon learning she was pregnant, she stopped smoking both cigarettes and marijuana and stopped drinking. She denied any other drug use. During the delivery, the baby experienced fetal distress owing to a nuchal cord, and the mother had to undergo an emergency C-section. Linda was born “blue” but did not require intubation. She was described as a colicky child until 4 months of age and experienced slightly below normal weight gain. She had normal developmental milestones but was always hyperactive and had temper tantrums that would last more than an hour as a toddler. With constant reminders, she responded to praise and rewarding good behavior during her prelatency years. Linda experienced severe separation anxiety when she went to preschool. When she began kindergarten, the mom states that the teacher loved her, but she often stated that she was shy at times and also easily distracted and could not seem to finish her work. She struggled with reading in first grade, but her grades did not become a problem until around third grade. At that time, she experienced a reoccurrence of her separation anxiety, accompanied with night terrors. When asked about physical or sexual abuse privately, she revealed she was sexually abused by a neighbor from age 7 to 9 but never told anyone because he threatened to hurt her family. The perpetrator moved away sometime within the past 6 months while she was in 11th grade in high school. As she approached middle school, she continued to struggle with her grades but became an average student. She entered high school and was a good athlete on the soccer team when her parents noted that the students she began to hang around with were skipping school. She quit the soccer team in the beginning of the 10th grade and began skipping school. During this same time period, her parents began to notice extreme mood swings. She would sneak out at night and was found by a neighbor asleep and drunk in their front yard. Her grades were close to failing, and she has been suspended three times for back talking the teachers and carrying cigarettes on campus.
Drug and Alcohol History
She reveals that she began experimenting with cigarettes at age 11. She now smokes about four to five cigarettes a day. She began drinking alcohol at the end of seventh grade (age 12). She noticed she was very shy and fearful of not being accepted and began getting drunk during 10th grade (age 15). She now uses to relax and “fit in” when using alcohol. Over the last 6 weeks, she has gotten drunk every weekend. She does not feel she has to use alcohol or marijuana and never uses when she has to study. However, she feels she cannot do well in school because her concentration is poor. She also began experimenting with marijuana during the summer between the 10th and 11th grades. She states that, when she began to drink, she noticed that she could drink more than her friends without getting drunk. She denies any other drug use.
Patient Medical History
There is no significant history except for childhood asthma.
Patient Psychiatric History
Though told by the school that Linda may need an evaluation for attention deficit hyperactivity disorder (ADHD), her mother did not want to put her daughter on medication and never requested her to be evaluated.
Family Medical History
There is a family history for liver problems in a paternal uncle who died of cirrhosis of the liver. He was a person/patient with alcohol dependence. Linda’s father has hypertension. Her paternal grandmother died of cancer when she was in the seventh grade. Linda was very close to her because she lived next door and was often at her house when her parents were at work.
Family Psychiatric History
Mom was very shy as a child and, as an adult, suffered from postpartum depression. She was treated with sertraline, which did relieve her depression. She restarted the medication over the past year owing to the stress she experienced with her daughter’s struggles. The uncle who died of cirrhosis of the liver had been diagnosed with posttraumatic stress disorder (PTSD) after serving in the Vietnam War. He was not treated for the PTSD and continued to drink. The dad’s father and mom’s father were persons/patients with alcohol dependence, and the mom states that dad’s drinking has increased recently to 6 to 10 beers every night.
Patient Social History
Linda states that she is sexually active but that she is promiscuous only when she is drunk. She does not know whether condoms were always used. Her last period was 2 weeks ago. She sought testing for a sexually transmitted disease and AIDS at a private clinic without her parents’ knowledge, and both came back negative. She would like to go to college but is not sure of whether she is smart enough to complete the 4 years required. She began to be attracted to a group of teenagers who were using alcohol. She then started dating a young man who introduced her to this group when she was in 10th grade. She lost interest in soccer and noticed she felt she belonged when “hanging around with them and using marijuana and alcohol” with this group. She has driven when intoxicated but has never used marijuana or alcohol alone. She reveals that, once while drunk, she got in a fight with a friend and hit her. She was never in trouble with the law prior to the present episode but admitted to having stolen beer with a friend from a local store. Linda was very ashamed about both stealing and her sexual activity when drunk. At times, when drunk, she could not remember what she had done. Though Linda feels she is spiraling out of control, repeated efforts by her parents to intervene have failed. Though she is unsure that she can quit drinking, she is willing to try. She wants to get back to sports. She did enjoy her youth group at church but is embarrassed about what she has done in the past and is afraid she would not fit in with this group now.
Family Social History
Linda is an only child. Mom finished high school and always had problems with math and reading. Dad was often in trouble for being the “class clown” and finished high school as an average student. There is no history of sexual or physical abuse in either of the parents. Dad has had problems keeping a job as a sales clerk because he is told he is too disorganized. This has put financial stress on the family. Mom works as a bank teller. They have always lived in the same area. There is no cultural diversity in the family. They are Catholic and, though they used to go to church, they have not been able to get their daughter to attend church with them since she got to high school. She was active in the church youth group prior to this. Mom and dad got married when they were just out of high school because mom got pregnant. They have separated on two occasions owing to dad’s drinking. This occurred when Linda was 8 years old and again when she was in the seventh grade. From the sixth grade to the present time, she has been a child who after school returned home alone until her parents got home from work.
Review of Systems
Linda states that it takes her 2 hours to fall asleep. She is wide awake and feels “like I could party all night.” She had recurring nightmares when the sexual abuse took place but not anymore. She feels fatigued lately and does not enjoy the things she used to enjoy. Her appetite has decreased, and she has lost 10 lb. She has a good body image and no signs of an eating disorder. She has no other physical symptoms but notices that she has difficulty getting up in front of the class to speak. She has had flashbacks of the sexual abuse she suffered from age 7 to 9 after she got drunk and engaged in sex with someone she did not know well. She is hypervigilant in the presence of older men. Overall, she gets much more irritated than she used to be. She cries almost daily, and, though she has never had a plan to kill herself, she feels so ashamed that she wishes she would not wake up. Her concentration at school is poor, and she gave up academically in middle school when she could not keep up.
Mental Status Exam
Linda’s affect is blunted, and she cried several times during the evaluation. She was well groomed, though wore no makeup. She appeared lethargic. She was underweight. Her judgment in the past was poor, but she seemed motivated to change her behavior. She was oriented, but her concentration was poor. She was unable to remember the three words given to her at the beginning of the mental status exam. She denied any auditory or visual hallucinations and had no delusional thinking. Her speech was slow but not tangential. She was articulate and seemed to appear brighter than the level suggested by her grades. As noted before, she denied suicidal plans but reported some ideation in the past.
Though Linda’s mother did not use a substantial amount of alcohol during pregnancy, it is important to mention the potentially severe effects of prenatal exposure to alcohol.
Prenatal Exposure to Alcohol
Though there is little research on the direct effects of substances of abuse on the brain reward circuitry that leads to addiction, research does show a high risk for later alcohol abuse in children exposed to alcohol prenatally (6). Areas affected by early alcohol exposure (which are discussed later in the chapter) lead to increased impulsivity that could promote increased risk for experimentation during adolescence.
Fetal alcohol syndrome (FAS) is the most common nonhereditary cause of mental retardation. The prevalence varies from 0.5 to 3 per 1,000 live births (7,8). Alcohol has become the most teratogenic drug in the United States. The incidence of FAS has shown a sixfold increase between 1979 and 1993 (9,10). This is primarily due to the fact that drinking has become more socially acceptable among women.
Children without the facial features commonly associated with FAS are referred to as having alcohol-related neu-rodevelopmental disorder (ARND). Unfortunately, without the facial features, it becomes more difficult to identify these children, and 60% to 90% escape identification in the normal population (11). The percentage of adolescents with FAS in juvenile justice forensic units is 3 to 10 times greater than the accepted worldwide incidence. The percentage of these youth with any alcohol-related diagnosis is 10 to 40 times greater than the accepted worldwide incidence (12). The cognitive and disruptive behavior problems in this population caused by exposure to alcohol prenatally may be one of the most undiagnosed reasons that lead to substance abuse and subsequent involvement with the juvenile justice system.
There are four cognitive areas affected by FAS/ARND: learning and memory (13–15), visual–spatial processes (14,16), executive function (13–15,17), and attention (18,19).
In another study, auditory processing problems were more impaired in FAS/ARND children than were visual processing problems. The latter information may account for another reason for attention problems with these children (20). Two studies on children with FAS/ARND demonstrated longer reaction times in these individuals. Therefore, children with FAS/ARND may have slower processing speeds that can be confused with attention problems (21,22). These studies stress the importance of evaluating every aspect of attention difficulties in FAS/ARND children.
Prenatal Exposure to Other Substances of Abuse
Though cocaine can cause developmental delays and LDs, it is clear that findings once thought to be specific to in utero cocaine exposure are more likely to be associated with alcohol, tobacco, and marijuana and the quality of the child’s environment (23). However, a better home environment was found to reduce many of the effects on lower IQ found at age 4 in cocaine-exposed babies (24).
Though the numbers of studies investigating the effect of prenatal caffeine exposure are few, there is little evidence at this time that prenatal caffeine has significant impact on childhood mental and motor development (25).
STRESSORS
Any compromise that would cause fetal distress or hypoxia increases the risk for the development of ADHD or LDs, which in turn could lead to an increased risk for substance abuse later in life. However, factors such as trauma, prenatal and postnatal stress, and early-life rearing experiences may alter addiction pathology later in life through changes in gene expression. These changes occur through chromatin remodeling without changes in DNA sequences (26–28). In our case scenario, a number of stressors could have increased the likelihood of substance abuse and subsequent addiction in this patient. These factors comprise prenatal exposure to substances of abuse, postnatal hypoxia and poor nutrition, the history of sexual abuse during childhood and again during adolescence, the parent’s separation, undetected LDs and ADHD and the associated stress of academic failure, and early substance use itself. The interplay between stressors in the environment and genes (otherwise known as epigenetics) is crucial to explore when considering processes that may increase the risk for developing substance abuse. Prenatal, postnatal, and physical/sexual abuse events that occur in childhood may cause an alteration in the expression of genes. These alterations may dysregulate the hypothalamic–pituitary axis (HPA), which, in turn, may result in an increased sensitivity to stress. The increased sensitivity to stress can increase the risk for using substances to relieve this stress. However, the use of substances to relieve stress also dysregulates the HPA, leading to a vicious cycle of worsening sensitivity to stress and substance use.
Adolescence is a transition period that is characterized by considerable neurobiologic changes and an associated increased propensity for substance use. It is also a time of enhanced sensitivity to stress. Therefore, the vicious circle described earlier may be further worsened during adolescence, leading to a steeper spiraling movement toward HPA dysregulation and severity of substance use problems. However, the road to addiction impacted by stress, environment, and developmental factors during adolescence may be modulated by individual genetic background. Studies have suggested an interaction between functional alleles that determine levels of neurotransmitter activity (e.g., dopamine, serotonin), environment (e.g., stress level, family factors), and severity of substance use problems (28). Certain environmental conditions could cause permanent changes in the neural circuitry that, in turn, could confer vulnerability for substance abuse and increase the risk of progression from substance abuse to addiction. These factors coupled with the family history of substance abuse may be the reason why Linda moved rapidly from one substance of abuse to another and to addiction.
Stress, Drugs, Reward, and the HPA: Koob’s Model–Antireward System
The antireward system involves the hypothalamic–pituitary axis (HPA) and norepinephrine (NE) in the brain stress/ emotional system and neuropeptide Y (NPY) in the anti-stress system (28). The role of these systems and the effect on the HPA will be discussed. First, most drugs have positive reinforcing effects. During acute drug use, most drugs of abuse increase dopamine in the shell of the nucleus accumbens (NAc), as well as other areas. Though the HPA can be activated by such things as stress, acute drug use can also activate the HPA. Both of these processes increase drug reward (29,30). The role of the prefrontal cortex (PFC) in modulating the NAc function will be discussed later. However, the system can still revert to normal or homeostasis if chronic drug use does not occur.
Chronic drug use can induce permanent changes in the motivation to use drugs. One theory that has attempted to explain how this occurs is the opponent-process theory (31). This theory proposes that an affective or hedonic habituation occurs (tolerance) at the same time as a negative affective response develops to abstinence (withdrawal). This would increase the motivation to use more drugs to (a) achieve the same effect and (b) ward off the negative effects of withdrawal. Another common finding that occurs during withdrawal, though not unique to it, is a hypofunctioning of the orbital frontal cortex (OFC). Other changes that occur during withdrawal include an increase in adrenocorticotropic hormone (ACTH), corticosterone, and corticotrophin-releasing factor (CRF), during over-activation of the HPA. Continued use leads to increased CRF and increasing anxiety each time the individual is abstinent.
Furthermore, overactivation of the HPA during chronic drug use also increases NE in the bed nucleus of the stria terminalis of the extended amygdala, which increases sensitivity to stress. Increases in ACTH, corticosterone, CRF, and NE are part of the recruitment of brain stress/emotion systems. In addition, during chronic drug use, NPY decreases in the central and medial nucleus of the extended amygdala. The change in NPY is referred to as a dysregulation of the brain antistress system. All these changes in CRF, NE, and NPY are referred to as the antireward system.
As these events occur, the individual becomes more sensitive to stress each time drugs are used, and, therefore, the individual is more likely to seek out drugs to relieve the stress. Each time drugs are used, the continued decrease in reward function in the brain reward system and the increased recruitment of the brain antireward system move the brain from a reversible state where homeostasis could have been reinstated to a more dysregulated state. This dysregulation occurs through a process known as allostasis. Allostasis is the attempt of the brain to achieve stability through change. Rather than the allostatic state reaching stability, it instead causes chronic pathologic states and damage. A change in baseline occurs such that environmental events that would normally elicit drug-seeking behavior have more impact; hence, the brain is more sensitive to stress-induced drug seeking (30), and this process involves input into the extended amygdala (28,29). Of course, as noted, if a person has had chronic stressful experiences, like the previously described sexual abuse experienced by Linda in her childhood, and subsequent PTSD symptomatology, this may cause an alteration on the expression of genes that may dysregulate the HPA, which would increase the sensitivity to stress and increase the risk for using substances to relieve this stress. Therefore, chronic stressors that occur before drug use occurs may set the stage so that the HPA system is less likely to return to normal once drug use and experimentation begin.
Changes in the Brain Reward Circuitry in the Transition to an Addicted State
Many teens who abuse substances will not develop an addiction after the maturation of the PFC in their early 20s (32). In those who develop addiction, a conceptualization of addiction neurobiology is helpful toward understanding the role that comorbidity and genes play. Certain comorbid conditions may affect the brain reward circuitry leading to self-medication of these comorbid conditions, or genes may be inherited that increase the sensitivity to the reinforcing effects of the drugs on the brain reward circuitry.
First, one must understand how an individual learns that a stimulus is salient so that the individual will learn to seek out the salient stimuli. When an individual engages in a pleasurable event, like eating ice cream, there is a rapid increase in dopamine in the shell of the NAc. Later, just the anticipation of eating the ice cream would cause an associated release of dopamine. In general, when this occurs, the shell of the NAc and dopamine are involved in initial reinforcement (29,33).
Using drugs that pass through the blood–brain barrier more quickly, like cocaine, would result in the same process stated above. However, when cocaine is taken intravenously, dopamine levels increase more rapidly and at higher levels, yielding a more pleasurable high then would occur if a normal salient stimulus, like ice cream, is taken orally. When cocaine is used in acute drug use, the same process is involved in using ice cream the first time. The shell of the NAc and dopamine are involved in this acute drug reinforcement. However, if the cocaine is used chronically, the PFC (anterior cingulate and the OFC) learns to put more salience on the use of cocaine and less salience on previously salient stimuli, like ice cream. A new circuitry becomes involved when this occurs. The core, not the shell, of the NAc; the basolateral amygdala; and the OFC are involved in chronic drug use that leads to addiction. The latter does not involve dopamine but rather the recruitment of glutamatergic efferents from the OFC to the core of the NAc (29,33).
What else causes the new circuitry to become involved during chronic drug use? Chronic drug use causes intracellular changes, leading to circuitry changes, resulting in dysregulation of the reward circuitry involving the PFC, basolateral amygdala, and the core of the NAc. Changes in the intracellular level have been described as three stages: acute, transition, and end/addiction stage (33).
Stage 1: Acute Drug Effects
After the acute administration of cocaine, dopamine levels of the NAc are elevated with little effect on glutamatergic tone, resulting in increasing locomotor activity and stimulating reward circuits (34,35). Moreover, the D1 dopamine receptor (DRD1) is stimulated, resulting in the following:
■ Activation of cAMP-dependent protein kinase (PKA)
■ PKA-induced phosphorylation of transcriptional regulator cAMP response element–binding protein (CREB)
■ Induction of early gene products such as cFOS
The second messenger cFOS causes short-term neuroplastic changes as the molecule cFOS is very unstable. This transcription factor turns on genes that produce dynorphin, which causes dysphoria during early drug withdrawal, and genes that inhibit dopamine and l c opioid receptors, which, in turn, decrease drug reward. This second messenger is so unstable that it dissipates in 4 to 12 hours. Therefore, limited exposure to drugs will allow the system to return to normal/homeostasis.
Stage 2: Transition to Addiction
Chronic repeated administration of a drug causes stimulation of DRD1 to produce proteins with long half-lives, such as Delta FosB. Delta FosB modulates transcription and synthesis of certain AMPA glutamate receptor subunits and cell signaling enzymes. A GluR1 glutamate receptor in the ventral tegmental area (VTA) forms after discontinuation of substances such as cocaine. Also, animals with activated Delta FosB have exaggerated sensitivity to the rewarding effects of drugs (34). In addition, Delta FosB also increases CdK5, which, in turn, blocks the stimulating effects of cocaine or blocks the anxiolytic effect of alcohol so that, in both instances, more cocaine or alcohol is needed to get the same effect (36). CdK5 may be one of the reasons tolerance occurs in substance abusers. Hence, if the foregoing is true, Delta FosB may increase the rewarding effects of drugs, causing an individual to seek out these rewarding effects more often and, when the individual does, more of the drug must be used to give the same rewarding effect.
Additionally, during chronic drug use and withdrawal periods, there is an increase of G protein binding AGS3. Increased AGS3 levels inhibit D2 receptor signaling and correspondingly increase D1 receptor signaling, which causes increased activity of projections from the PFC (in this case, the anterior cingulate and the OFC) to the core of the NAc, which mediates behavior. What do these changes mean? The PFC is involved in salience (see Chapter 1). However, these changes reduce the salience of nondrug motivational stimuli so that normal stimuli such as food are less salient. The PFC becomes hypoactive to previously salient stimuli. However, when drug-associated drug stimuli are available, there is a profound activation of the PFC and glutamatergic drive to the core of NAc, and drug craving occurs. The changes in determining what is now salient (drugs) and the activation of the PFC to the core of the NAc to produce craving move the brain from a transitional stage to the end stage of addiction. As previously mentioned, glutamate plays a more important role in drug seeking after chronic drug exposure versus dopamine playing a role in acute drug use (drug taking, reinforcement). The NAc core increases release of glutamate in response to stimuli that induce drug seeking and intake. Such stimuli may be a cue previously associated with drug use or a mild stressor.
End-Stage Addiction
Changes in protein expression that mediate the transition to addiction may induce changes in protein expression that move from temporary and reversible to permanent.
What effect does glutamate produced by the NAc have on permanent adaptations that lead to continued drug use? Two things occur. First, when presynaptic glutamate is released, the GluR2/3 inhibitory autoreceptor becomes less effective. Less glutamate is released in the NAc after cocaine withdrawal and, therefore, presynaptic inhibitory GluR2/3 tone is decreased, and more glutamate is released in the core of the NAc when a mild stressor occurs or a cue associated with drug use occurs. Increase in glutamate postsynaptically causes an increase in proteins that cause rigid dendritic morphology and signaling. The changes that occur after chronic drug use are more permanent than changes that occur during acute drug use and may be central toward explaining the transition from drug taking, to addiction, and eventually relapse. All relapses can be categorized as due to (a) drug/ behavior reexposure, (b) cue, or (c) stress.
The amygdala is involved in attaching affective valence to stimuli and has thus been implicated in relapse of all three types mentioned earlier. The basolateral amygdala recognizes motivationally relevant events, triggering the PFC to determine salience, and the PFC then influences the NAc, which further mediates behavior (29,33). Hence, this process may reflect a clinical triad that is the phenotype of addiction: (a) loss of control (when the patient continues to use even though there is no enjoyment is using), (b) continued use despite negative consequences, and (c) preoccupation with the drug. The changes that occur after chronic drug use are believed more permanent than changes that occur during acute drug use. Adolescent brains not only may be remodeled by this state-dependent process involved in the transition from drug taking (or perhaps substance abuse) to drug addiction (substance dependence) but also may have trait-dependent, preexisting vulnerabilities in these brain systems. As adolescents may not be able to differentiate between motivationally relevant events (see developmental effects further), when addiction occurs, the PFC may increase the behavior to seek out risky behaviors whether they are relevant or not, or addiction may tune the otherwise more sensitive amygdala found during adolescence into a more sensitive drug-state amygdala that now seeks out only drug-associated relevant events once drug use begins.
This new allostatic state involved in the transition to addiction involves a decreased reward circuitry and increased antireward circuitry. As noted in Figure 102-2, cues would affect the drive to seek out drugs in the VTA and the basolateral amygdala that affect changes in drug circuitry, but changes in the antireward system (that would affect seeking out drugs to relieve stress) would occur in the extended amygdala (28,33).
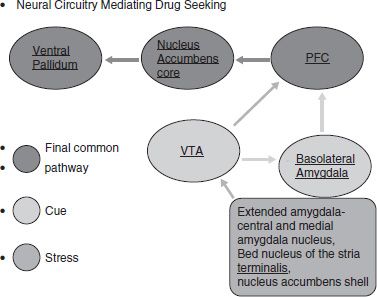
FIGURE 102-2 Role of the reward circuitry and antireward circuitry from a developmental perspective. (Adapted from Koob G, LeMoal M. Addiction and the antireward system. Annu Rev Psychol 2008;59:29–53.)
Role of Genes
As noted, stressors can turn on genes that can lead to the dysregulation of the HPA. In fact, high alcohol-preferring rats that have increased anxiety-like responses have been shown to have lower NPY activity, which is involved in the antireward system. However, they also have decreased dopaminergic activity. The number of dopamine receptors genetically inherited may play another role in genetic vulnerability. As noted previously, lower D2 receptors may influence PFC so that it no longer recognizes normal reinforcers as salient. The role of D2 receptors may also influence compulsion. When D2 receptors are decreased in the NAc, there is a corresponding decrease in metabolism in the orbital frontal gyrus and the cingulate gyrus. The cingulate gyrus initiates the ability to restrain control, and the orbital frontal gyrus shifts attention to what is salient. If the orbital frontal gyrus is destroyed, Volkow et al. (37) believe the drug abuser will continue to use drugs, even if using them is no longer pleasurable. Therefore, if decreased D2 receptors result in decreased metabolism in the cingulate gyrus (so it can no longer inhibit the drive to use drugs) and the orbital frontal gyrus (so that it continues to compulsively use what it sees as salient, drugs, even though it is no longer pleasurable to do so), a person who has inherited decreased D2 receptors would be at more risk for developing SUD. In fact, Volkow et al. (38,39) have shown on PET imaging that nonalcoholic family members in alcoholic families had higher than normal D2 receptor levels in the caudate and ventral striatum and metabolism in the anterior cingulate (Brodmann area 24/25), orbitofrontal (Brodmann area 11), and the PFC (Brodmann area 9/10). These individuals also had personality scores of positive emotionality on the Minnesota Multiphasic Personality Inventory (MMPI). This suggests that higher D2 receptor levels could protect against alcoholism by regulating circuits involved in inhibiting behavioral responses and in controlling emotions. To further illustrate this, Thanos et al. (40) increased D2 receptors in mice (by using an adenovirus); alcohol consumption by the mice decreased by 70%. Therefore, people born with an increase in D2 receptors may be at less risk to develop SUD, and those who inherit a decrease in D2 receptors may be more vulnerable. The latter may have been the type of genetic makeup inherited by the young woman discussed in the case scenario.
Couple the decreased D2 receptors with the changes that occur in the motivational circuitry during adolescence (see developmental vulnerability further), and it becomes clear why exposure to substances of abuse during adolescence may increase the risk for developing substance abuse.
Other inherited receptors, along with inherited susceptibility to motivational components, may increase the risk of SUD. Two fatty acid derivatives characterized to be arachido-nylethanolamide and 2-arachidonylglycerol both have been isolated from both nervous and peripheral tissues. Both these compounds have been shown to mimic the pharmacologic and behavioral effects of delta-9-tetrahydrocannabinol, the psychoactive component of marijuana. Studies by Hungund and Basavarajappa (41) demonstrated the downregulation of CB1 receptor function and its signal transduction by chronic alcohol use. The observed downregulation of CB1 receptor binding results from the persistent stimulation of receptors by the endogenous CB1 receptor agonists arachidonyletha-nolamide and 2-arachidonylglycerol, the synthesis of which is increased by chronic alcohol use. The deletion of CB1 receptor has recently been shown to block voluntary alcohol intake in mice, which is consistent with the authors’ previous findings where the DBA/2 mice known to avoid alcohol intake had significantly reduced brain CB1 receptor function. These findings suggest a role for the CB1 receptor gene in excessive alcohol drinking behavior and development of alcoholism.
In addition, just as increased D2 receptors may decrease the rewarding effects of drugs during acute drug use and, therefore, decrease the risk of addiction, knockout of the mu opioid receptor studied in mice may influence the risk of developing SUD. Mu knockout mice demonstrated not only a decrease in heroin self-administration but also a decreased rewarding effect of alcohol, nicotine, and cannabinoid (42). These changes cause a negative motivational state during acute abstinence and increase the reaction to stress so that relapse is more probable.
Several representative genes that may be associated with an increased risk for substance use disorders include a lack of a beta-2 subunit on the nicotine receptor, which caused decreases in self-administration (43), and decreased in D1 receptor availability, which caused a decrease in cocaine self-administration in mice (44). Also, a gene encoding the transcription factor FosB/Delta FosB has been found to be associated with high novelty seeking and addiction susceptibility in animals (45).
A gene that has been linked to externalizing disorders has been found for the alpha-receptor subunit of the neurotransmitter GABA located on chromosome 4. It is also related to alcohol and drug dependence (46).
Genetic architecture of addiction has also been studied by Uhl (47). Uhl found 15 small chromosome regions, called “rSA,” that contained markers found in multiple studies of addiction to legal and/or illegal substances. Molecular pathways that help maintain the addiction state have been identified that could disrupt or enhance the development of addiction owing to specific genetic variability in these pathways. For instance, a core component of addiction-induced adaptations in glutamatergic transmission has been identified as CAMKII. This component may play a role in the morphologic changes and memory circuits triggered by addictive drugs. Disruption of CAMKII impaired the stabilization of synaptic plasticity and memory consolidation caused by addictive drugs. GnRH, which may play a role in certain emotional behaviors associated with the HPA during stress-induced drug seeking, has also been identified (48). Though much more research is needed to further understand the molecular genetics of addiction, additional pharmacotherapies will be discovered.
STUDIES OF INHERITABILITY
In the case study example, Linda’s father had a family history of alcoholism. Adoption studies have shown increased risk for alcoholism of adopted-away children of persons/ patients with alcohol dependence (49) and increased risk for substance abuse other than alcohol in adopted-away studies (50). However, alcohol use by adoptive parents did not increase the risk for alcohol abuse in adoptive children (51).
Adoption studies have shown that genetic susceptibility seemed to be a stronger predictor of risk for substance abuse than exposure to adoptive parents using substances. However, both genetic and environmental influences may be correlated to substance initiation, whereas progression to substance abuse and dependence may be more related to genetic factors alone. In adoption studies conducted by Kendler and Presscott (52), 485 monozygotic and 335 dizygotic twins demonstrated that cannabis use was influenced by genetic and familial environmental factors, whereas cannabis abuse and dependence were solely related to genetic factors. This was also true for cocaine use versus abuse and dependence (53). Schuckit (54) has shown greater tolerance in children of persons/patients with alcohol dependence. In his study, children of persons/patients with alcohol dependence had to use greater proportions of alcohol before the reflex response to a stimulus was delayed to the same degree found in responses of children of nonalcoholics. In children of nonalcoholics, reflex response to a stimulus was delayed to the same degree on lower portions of alcohol. This diminished response to alcohol was also measured by subjective feelings, levels of body sway, electrophysiologic functioning, and change in three hormones.
Personality, Drug History, and Comorbidity
Though Koob and LeMoal (28) state that personality, drug history, and comorbidity are more likely to influence drug use later, they all have some root in early childhood and adolescent substance use disorders. Seldom does any patient with a SUD develop it without some significant precursors in their developmental history, and psychiatric comorbidity may increase the risk or speed of transition from substance use to SUD.
Drug History
Addiction to drugs and alcohol can occur anytime throughout the life of an individual. However, age is a risk factor likely to influence the onset of substance use during childhood and adolescence. In this case scenario, the patient began using cigarettes at age 11, began drinking alcohol at age 12, and began getting drunk at age 15. She began using marijuana at the end of 10th grade. She began getting drunk every weekend as she entered 11th grade. A study of youngsters who began drinking at an early age, 11 to 12 years of age, had a higher probability of meeting the DSM-III-R criteria for substance abuse (13.5%) and substance dependence (15.9%) as compared to those who began drinking at age 13 or 14 (13.7% and 9.0%, respectively). Those who drank at age 19 or 20 had rates of 2% and 1%, respectively (55). Schuckit et al. (55) have noted that the age where a patient with substance abuse was most likely to have started drinking was 13, when first drunk was age 15, had their first problem associated with drinking at age 18, and first dependence was age 25 to 40. Death was most likely to occur by age 60. Important is that rapid progression of SUD occurred often with earlier age of onset and frequency, not duration of use (56,57). Those individuals with earlier onset had a shorter time span from first exposure to addiction than did adult-onset groups (58). Age of onset of heavy drinking also predicted alcohol-related problems (59). Early age of onset also influences higher risks for the use of other substances, as noted in this case scenario. Adolescent-onset adults had higher lifetime rates of cannabis and hallucinogen use disorders, shorter times between the development of their first and second dependence diagnosis, and higher rates of disruptive behaviors and major depression (58). This patient began sneaking out during 10th grade, and her review of systems and mental status indicated that she may have a major depressive disorder.
Screening the patient with the CRAFFT (60) indicates possible substance abuse, possibly substance dependence. Her unidentified and untreated ADHD, PTSD, LDs, and possible major depressive disorder may be factors that are contributing to her persistent use. She does not compulsively use but states she uses now to relax and “fit in” and relieve stress; thus, her substance use may be affecting her HPA, making her more sensitive to stress and more likely to use to relieve the stress. As noted before, impulsive use implies possible PFC hypofunctioning whereby the patient may prioritize salience to drug-related stimuli over natural reinforcers. It is difficult to determine whether she is prioritizing drugs as salient because of comorbid conditions, like depression. Anhedonia can diminish pleasure in everyday circumstances, but drug use can also cause anhedonia. It is also difficult to determine whether she is using drugs to ward off the negative effects during withdrawal, such as anxiety, because she has an untreated PTSD and social phobia. Treatment of these conditions, ideally in parallel, may elucidate the salience.
Personality
Temperament may explain why some adults continue to demonstrate characteristics of dependence. Both Cloninger (51) and Babor (61) identified personality traits associated with negative prognosis. Cloninger’s type 2 and Babor’s type B persons/patients with alcohol dependence share common characteristics: early onset of spontaneous alcohol-seeking behavior, diagnosis during adolescence, rapid course of onset, presence of genetic risk factors, deviancy, and greater psychological vulnerability. Cloninger’s type 2 and Babor’s type B persons/patients with alcohol dependence may be related to youth who are thought to have conduct disorder. Conduct disorder is thought to be related to genetic vulnerability and negative environmental factors (poverty, parental neglect, marital discord, parental illness, and/or parental alcoholism) and is associated with impairment in frontal lobe function, affecting the ability to plan, to avoid harm, and to learn from negative consequences—traits often found in the type 2 or type B persons/patients with alcohol dependence. The same type of personality characteristics was found in kindergartners who had an increased risk for development of SUD in adolescence (62).
More recent research has tried to describe the relationship between personality and risk-taking behaviors in six areas, including smoking, drinking, drugs, sex, driving, and gambling (63). Risk taking across all six areas was related to impulsivity, sensation seeking, aggression, and sociability but not to neuroticism–anxiety and activity.
Though the patient in this case scenario seemed to have many of the characteristics that are found in Cloninger’s type 2 and Babor’s type B personality types (early-onset alcohol-seeking behavior, genetic precursors or family history of alcoholism, marital discord, and rapid course of onset of substance abuse), her early personality traits did not suggest an early-onset conduct disorder that would offer a more negative prognosis. In fact, she seems to be very motivated for treatment (64) and did not seem to show any evidence for sensation seeking, aggression (except one time while drunk), or sociability, which would increase her risk taking. Her impulsivity seemed to be related to an untreated ADHD. Her history of separation anxiety and possible social phobia may have led to her using in order to relax and socialize. Her anxiety would have put her at less risk for risk taking. In fact, she was embarrassed by her behavior, especially her history of rape under the influence of alcohol. She is motivated to try to quit abusing substances. Therefore, this patient seemed to be a good candidate for treatment.
Comorbidity
Any psychiatric disorders that are not recognized and treated during any developmental stage can increase the risk of substance abuse. The chapters in this text that discuss comorbidity and risk factors will illustrate this. However, there are a few psychiatric disorders that are worth mentioning, which, if untreated, will increase the risk for substance abuse perhaps by making the brain neurobiologically more vulnerable to the development of substance abuse. Therefore, these disorders can be classified as a developmental vulnerability. They are mentioned here briefly. Academic failure and learning disorders are not often given sufficient discussion and, therefore, also are elaborated. Also, as many neuropsychiatric disorders can affect executive function, this may interfere with and undermine treatment. Bolla et al. (65) have shown that abstinent cocaine users show less activation than nondrug users in the left anterior cingulated cortex (ACC) and the right lateral prefrontal cortex (LPFC) and greater activation in the right ACC. All of these findings may impair executive function and further affect decision making once substance use begins, especially if the deficit in executive functioning occurs in undetected and untreated psychiatric disorders.
Mood and Conduct Disorders
Though ADHD, major depression, and conduct disorder may be important components of substance dependence, depression may be the primary variable related to substance use disorder in women at any age (66). Though girls are more likely to have internalizing disorders, a study by Couwenbergh et al. (67) has shown that externalizing disorders, especially in boys, were consistently linked to SUDs in treatment-seeking adolescents. Those with conduct disorders had a higher risk of ending up in a juvenile justice system. However, depression in boys may be linked to earlier onset of conduct disorder. In a study of adolescent deviant boys with conduct disorder and comorbid substance abuse reported by Riggs and Whitmore (68), depressed boys were more likely to have ADHD, PTSD, anxiety disorder, and an earlier onset of conduct disorder when compared to nondepressed boys. In this same study, the depressive symptoms did not seem to be relieved after 4 weeks of abstinence.
Hypofunctioning of the OFC and the anterior cingulate has been shown to occur in depressed untreated patients. Hypofunctioning of the PFC (OFC and anterior cingulate) in depressed patients may cause disinhibition of the PFC to the amygdala, thereby correspondingly causing a disinhibition of projections to the hypothalamus. In turn, there would be an increase in CRF. The latter would increase anxiety symptoms and may play a role initially in explaining why people who are depressed “self-medicate.” However, during chronic drug use, this may exacerbate the antireward system, which may also increase the use of substances to relieve anxiety (69,70).
Rao et al. (71) identified a unique feature of girls with conduct disorder, SUD, and depression. These girls had more anxiety disorders and elevated cortisol near sleep onset (when the hypothalamic–pituitary system is expected to be more active) than depressed girls without SUD. It is felt that the use of alcohol and/or stress in these girls may be the reason why cortisol levels are much higher than in girls with conduct disorder and depression alone. The role that this may play in treatment, if any, is unclear at this time. However, the role of cortisol (glucocorticosteroids) can influence dopamine transmission in the ventral striatum and the shell part of the NAc and thus may drive the CRF system (28). This may affect the antireward system and increase the likelihood of transition to addiction.
One study has examined the risk of SUD independent of the diagnosis of conduct disorder in late-onset bipolar disorder. Wilens et al. (72) have reported that those with adolescent-onset bipolar disorder had an 8.8 times greater risk of developing substance use disorder than those with childhood-onset bipolar disorder, and no other disorder, including conduct disorder, accounted for the risk. This finding may have something to do with the maturation of the NAc that seems to occur at a faster rate than the PFC during adolescents (73). This would give greater weight to the intensity of the rewarding effects when using substances during adolescents, especially for youth who have bipolar disorder where they may seek out risky behaviors more often than their adolescent counterparts who do not have bipolar disorder.
Though patients may use substances to self-medicate their manic symptoms, it is clear that the use of multiple substances, such as marijuana and alcohol, may, in reality, make the neurobiologic effect even worse. When patients (aged 18 to 65 years) presented with active marijuana and alcohol use in the manic phase, the marijuana did not decrease the level of the manic state. Thus, though the sensation of feeling calmer may have been experienced by bipolar substance abusers who were manic and using alcohol, the mania symptoms were actually worse in those who presented with bipolar disorder and marijuana and alcohol use than in those with bipolar disorder and alcohol use alone. Use of multiple substances may be a sign of a more progressive addiction, which would likely decrease the ability to inhibit compulsive behavior and the level of mania. Moreover, the type of treatment used may have less effect on those who are actively using marijuana during their presentation with mania and alcohol. In the latter group, those who were treated with lithium and psychosocial therapy (compared with those treated with lithium, valproic acid, or psychosocial treatment alone) had the highest percentage of heavy drinking days as compared to those with bipolar disorder who use alcohol alone (74). The same effect has been studied in adolescents. Geller et al. (75) have shown that bipolar adolescents who also used alcohol had a decrease in manic symptoms and alcohol use when treated with lithium.
Anxiety Disorders
Teachers and clinicians will often not recognize children with social phobia because they do not necessarily present with behavioral problems. However, those who are recognized owing to their aggressiveness should also be evaluated for shyness. In one study by Swan (76), the combination of shyness and aggressiveness in boys was a more valid predictor of future cocaine use than a history of aggressiveness alone.
The role of dopamine in the development of social anxiety may help to explain the increased risk of developing substance abuse if social phobia goes untreated. Striatal dopamine reuptake sites were markedly lower in patients with social phobia as compared to controls (77), and there was lower binding of D2 receptors in the striatum of untreated social phobia patients as compared to controls (78). The young woman in the case scenario may have had lower D2 receptors not only owing to her family history for substance abuse but because of her untreated social phobia.
One study of juvenile justice–involved adolescents noted that 11% of juvenile detainees met criteria for PTSD and that more than half had witnessed violence that precipitated their trauma (79). Two direct relationships have been found between childhood trauma and exposure and adult criminal behavior in women. Having been in foster care or adopted was positively related to engaging in sex associated with prostitution. However, early experiences of traumatic events (i.e., death of close relative, serious accidents) were related to engaging violent crime. For African American as well as white women offenders, childhood traumatic events were related to the development of adolescent substance abuse (80). This may help correlate the relationship of stress and its effect on the HPA, which may increase the levels of CRF, ACTH, and cortisol. If a stressor is mild and stimulates the HPA to release CRF and then ACTH, cortisol levels rise in response to ACTH. When cortisol reaches the pituitary gland, it inhibits further release of CRF and ACTH. However, if the stressor is intense; signals in the brain for more CRF outweigh the inhibitory effects of cortisol. In this latter situation, there may be a correspondingly increased risk to use substances of abuse to relieve the anxiety associated with the heightened CRF levels.
The role of the OFC in anxiety disorders correlates to treatment response to cognitive–behavioral therapy (CBT) and medications. For example, the high magnitude of response of the OFC in functional magnetic resonance imaging (fMRI) studies prior to treatment inversely correlated with the response to medications, but the high magnitude of the response of the OFC directly correlated with the response to CBT. If the OFC is hypofunctioning in an individual with an anxiety disorder and an SUD, the patient may not respond to CBT treatment for the anxiety disorder as well as CBT treatment for the substance abuse (81,82).
Attention Deficit Hyperactivity Disorder
It is well recognized that ADHD with conduct disorder has a much greater risk for developing substance abuse than ADHD alone. In fact, in a twin study done by Disney et al. (83) of 626 pairs of 17-year-old twins, ADHD did not increase the risk for substance abuse unless it was associated with a co-occurring conduct disorder. Biederman et al. (84) have shown that untreated ADHD has more risk for future substance abuse than ADHD that is treated. If an adolescent has ADHD and is not treated, the risk of developing substance use disorder is two times higher than in those who have ADHD and were treated with stimulants. The role of stimulants in the treatment of ADHD and possible explanations for the decreased risk for substance abuse is discussed further. However, untreated ADHD seems to involve an underactive anterior cingulate and PFC (85,86). The role of the anterior cingulate and PFC in inhibiting impulsivity found with ADHD patients could increase the risk of using substances of abuse and lead to substance abuse and addiction.
Exposure to Stimulants for Treatment of Attention Deficit Hyperactivity Disorder and Attention Deficit Disorder
As there is so much controversy over the use of stimulants in the treatment of ADHD and attention deficit disorder (ADD), a common pediatric disease, some point of clarification between the uses of stimulants for medicinal versus recreational drug use should be noted.
First and foremost, one must consider the speed with which substances of abuse move through the blood–brain barrier. Swanson and Volkow (87) have pointed out that the liability of a drug to cause reinforcing acute euphoric feelings is associated with the instant high achieved by using drugs of abuse by smoking, snorting, or using intravenously. There is a rapid dopamine blockade of dopamine transporters in the ventral striatum (containing the shell of the NAc) causing a euphoric high. However, methylphenidate taken orally does not produce this rapid high because it enters the brain barrier more slowly and is less associated with a high that causes a reinforcing effect of the drug. However, many have postulated that even oral use of stimulants may result in dopamine system alterations, which in turn may increase sensitivity to the reinforcing effects of the drug and, hence, increase the risk of later substance abuse. However, initiation of methylphenidate at an earlier age for the treatment of ADHD was not associated with the development of substance abuse and may be protective (88).
There are other studies that may explain why early use of stimulants for the treatment of ADHD may decrease the risk of developing substance abuse. For instance, this may be explained by research done by Castellanos et al. (89). Castellanos reviewed total cerebral volume of treated and untreated adolescents with ADHD. Total white matter in the unmedicated ADHD adolescents was lower than medicated and normals. It is hypothesized that perhaps the trophic effect on myelination, dendritic branching, and length of spines in the treated ADHD youth was somehow protective, perhaps by providing a greater brain functional reserve that may be associated with a decreased risk of substance use disorder. In addition, Luna and Sweeney (90) have shown that a normal process that occurs during adolescence is an increase in myelination. The effect is an increase in processing speed. Therefore, the lack of myelination may decrease processing speed, and these effects may increase the risk for substance abuse related to poor academic success.
Research by Thanos et al. (91) may give further explanation for the role between early use of stimulants for medicinal reasons and decreased risk for development of substance abuse. As noted before, overexpression of D2 receptors reduces alcohol and cocaine self-administration in mice (39,40,92) and decreases drug liking and may be protective against substance abuse in humans (38,41). Thanos found significantly reduced rates of cocaine self-stimulation during adulthood in periadolescent rats treated with 2 mg/kg oral methylphenidate for 8 months as compared to periadolescent rats treated with 1 mg/kg or rats receiving water. The availability of D2 receptors was significantly lower after 2 months of treatment in rats given 1 or 2 mg/kg of methylphenidate compared with control rats, but after 8 months of treatment, it was significantly higher. The rats given 2 mg/kg of methylphenidate at 8 months had greater D2 receptor–binding availability than rats given 1 mg/kg. Therefore, in rodent studies, consistent methylphenidate treatment (started in adolescence) attenuated cocaine self-administration during adulthood. In the case scenario presented here, if this patient had been treated for her ADHD early on, she may not have lost so much potential academically in school and this may have reduced the risk for the development of substance abuse.
Learning Disorders
Academic failure and low commitment to school are other psychobehavioral factors associated with influencing drug use during childhood and adolescence. Beyond early onset of use, poor academic achievement, poor social skills and competence, LDs, and poor self-esteem were found to be related to drug abuse (93,94). Hops et al. (95) found that substance abuse at age 14 or 15 could be predicted by academic and social behavior between the ages of 7 and 9. Too often, ADHD is assumed to be the reason for school problems, and a comorbid LD goes undetected. Simkin (96) tested ADHD/ ADD children who had responded well to stimulants in elementary school but later began to struggle in middle or high school. Of those tested, 77 children had ADD/ADHD alone or ADD/ADHD with a comorbid anxiety and/or depression, which were well controlled for. Of these children, the average IQ was 122. Ninety-two percent were found to have an auditory processing problem, 70% had a processing speed problem (on the cognitive battery of the Woodcock Johnson-III), and 87% had a disorder of written expression (on the Woodcock Johnson-III). This is extremely important as ADD/ADHD can be confused with processing problems. Tapert et al. (97) found that adolescents with attention difficulties predicted substance abuse and dependency 8 years later. This study controlled for substance involvement, education, conduct disorder, family history of substance abuse, and LDs. The attention difficulties were not necessarily related to ADHD/ADD. Given the foregoing information, the young woman in the case scenario should be evaluated not only for ADHD but also for an LD. This young woman may have had a reading disorder, and proper educational testing should be performed during her evaluation and treatment. Fortunately, this young woman did not end up in a juvenile justice system. However, for youth who do present to a juvenile justice system, these adolescents experience much higher rates of psychiatric disorders than do adolescents in the general population (98). However, what brings them to the attention of the juvenile justice system is an often co-occurring SUD. Many of these individuals also have a comorbid LD. In a 2002 report on the California Juvenile Justice system, a pathway was described that denotes how children, beginning in the school system, are identified for risk factors, but no appropriate comprehensive evaluation is sufficiently done to prevent entrance into the juvenile justice system (99). First, the child may be identified as having a mental health need at age 5 by a teacher. A referral is made to special education by age 7. Then, the child may interact with the mental health and child welfare services by age 9, and inpatient hospitalization may occur by age 12. The pathway ends by entrance into the juvenile justice system by age 14. Windows of opportunities for proper evaluation and intervention are missed along the way. For instance, before a referral to special education occurs, an evaluation for psychiatric disorders should occur or learning difficulties like dyslexia should be identified to reduce the risk of entering the juvenile justice system. In fact, in a study that looked at young offenders and dyslexia, 50% were dyslexic (100).
Early detection of LDs is essential to prevent the increased risk for developing substance abuse. However, the timing of the intervention may also be crucial. Wright (101) has suggested that processing problems associated with language impairment, dyslexia, and auditory processing problems is a developmental delay that if not corrected before age 10 may become permanent during adolescence. This does not mean that interventions done after age 9 will not be helpful; however, they may not be as robust since the brain may not be as plastic and responsive to interventions. Therefore, if these disorders are not detected and intervened upon at the appropriate time, a window of opportunity may be lost. Stimulants may help ADD/ADHD but, should LDs be detected, accommodations must be made in order to ensure continued success. Early detection of processing problems should occur whenever any child has difficulties with reading in order to prevent the risk of developing substance abuse and involvement with the juvenile justice system.
Schizophrenia
In a study by Hambrecht and Hafner (102), a vulnerability hypothesis was constructed to help explain the frequent use of marijuana in patients with schizophrenia. This vulnerability hypothesis was divided into three groups. Frequent use in group 1 may have decreased the threshold for the appearance of schizophrenia as they had used for several years before the onset of the disorder. Group 2 may be made up of a vulnerable group in which the dopaminergic stress factor may precipitate the onset of schizophrenia. This group developed the onset of schizophrenia in the same month they began to use marijuana. Group 3 may use the marijuana to self-medicate, because they developed the onset of marijuana abuse after the onset of the schizophrenia. This vulnerability hypothesis may explain the effects that other substances of abuse may have on the developing brain and how other psychiatric disorders and/or learning disorders may emerge or interplay with drugs of abuse. More research is needed in regard to this hypothesis.
DEVELOPMENT OF THE ADOLESCENT BRAIN
Obviously, if psychiatric disorders and learning disorders are detected early and treated, there is less risk for developing substance abuse. However, adolescence is a time of experimentation. Why do adolescents experiment with risky behaviors? Much of this may be due to the dramatic changes that occur during adolescence. The following is an explanation of these changes.
Though the literature suggests that adolescent cognitive development is progressively increasing during adolescence and that this cognitive control capacity is positively associated with maturation or increased activity within the PFC (103–105), adolescence also has shown to be a developmental stage associated with suboptimal choices. Therefore, adolescence seems to demonstrate a nonlinear change in behavior unexpected from what the previous research has illustrated. If cognitive control and an immature PFC were the basis for suboptimal choice behavior, then children should look remarkably similar or even worse than adolescents, given their less developed PFC and cognitive abilities. Thus, immature prefrontal function alone cannot account for adolescent behavior.
It has been suggested that perhaps researchers should consider adolescence as a period wherein two separate entities are independently working: lack of cognitive control and risk taking. Focusing on these two entities from a neurobiologic level with two different trajectories may give more insight to the reasons behind adolescent behavior (Casey et al., unpublished). Using recent imaging studies of adolescence (73,106,107), Figure 102-3 depicts this model. On the left (A) is the traditional characterization of adolescence as related almost exclusively to the immaturity of the PFC. On the right (B) is our proposed neurobiologic model that illustrates how limbic subcortical and prefrontal top-down control regions must be considered together. The cartoon illustrates different developmental trajectories for these systems, with limbic systems developing earlier than prefrontal control regions. According to this model, the individual is biased more by functionally mature limbic regions during adolescence (i.e., imbalance of limbic relative to prefrontal control) as compared to children, for whom these systems (i.e., limbic and prefrontal) are both still developing, and compared to adults, for whom these systems are fully mature. Further, the model reconciles the contradiction of health statistics of risky behavior during adolescence, with the astute observation by Reyna and Farley (108) that adolescents are able to reason and understand the risks of behaviors in which they engage. According to the model in Figure 102-3, in emotionally salient situations, the limbic system will win over control systems given its maturity relative to the prefrontal control system.
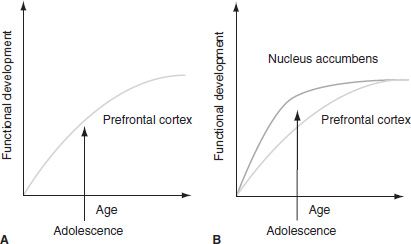
FIGURE 102-3 A: The traditional explanation of adolescent behavior has been suggested to be due to the protracted development of the prefrontal cortex. B: Our model takes into consideration the development of the prefrontal cortex together with subcortical limbic regions (e.g., nucleus accumbens) that have been implicated in risky choices and actions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


