, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Approximately 60 million people in the United States have hypertension. Many are undiagnosed or untreated. Different populations have different risks and different consequences of hypertension. Increased hypertension is seen with aging, positive family history, African-American race, and exogenous factors such as smoking. Although African-Americans make up only 12 % of the US population, they are fivefold overrepresented among patients with end-stage renal disease (ESRD) presumed due to hypertension [1, 2]. Hypertension is associated with significant morbidity and mortality due both to cardiovascular and renal diseases [1–5].
Arterionephrosclerosis
Introduction/Clinical Setting
Approximately 60 million people in the United States have hypertension. Many are undiagnosed or untreated. Different populations have different risks and different consequences of hypertension. Increased hypertension is seen with aging, positive family history, African-American race, and exogenous factors such as smoking. Although African-Americans make up only 12 % of the US population, they are fivefold overrepresented among patients with end-stage renal disease (ESRD) presumed due to hypertension [1, 2]. Hypertension is associated with significant morbidity and mortality due both to cardiovascular and renal diseases [1–5].
Essential hypertension is diagnosed when no cause is found. Hypertension may also be secondary to various hormonal abnormalities, including excess aldosterone, norepinephrine, or epinephrine, or produced from adrenal cortical, medullary, or other tumors; renin-producing tumors; or hypercalcemia or hyperparathyroidism. Other secondary causes include neurogenic, iatrogenic, and structural lesions (e.g., coarctation of the aorta).
Renal hypertension refers to hypertension secondary to renal disease. Chronic renal disease is the most common form of secondary hypertension (5–6 % of all hypertension). The kidneys modulate blood pressure in several ways: They modulate salt/water balance under the influence of aldosterone. The kidney is also a major site of renin production, which allows generation of angiotensin II, an important vasoconstrictor and stimulus for aldosterone secretion. In renovascular disease (i.e., stenosis of the renal artery), renal ischemia is thought to be the stimulus that increases renin-angiotensin system activity, thereby increasing systemic blood pressure. In renal parenchymal disease, multiple factors contribute to increased blood pressure. The decreased mass of functioning nephrons leads to a decrease in the glomerular filtration rate (GFR), leading to increased extracellular volume and increased angiotensin, aldosterone, and other vasoactive substances.
The most common complications in untreated hypertension are cardiac, renal, and retinal disease. Half of hypertensive patients die of cardiac disease, 10–15 % of cerebrovascular disease, and about 10 % of kidney failure. Treatment to decrease blood pressure reduces mortality and especially reduces the incidence of cerebrovascular accidents. Hypertension accelerates the decline in GFR characteristic of many chronic kidney diseases, whether the primary cause is hypertension associated or not. Chronic kidney disease is common, affecting 195,000 Americans, with 45,000 new patients enrolled in end-stage treatment Medicare programs yearly. It has been postulated that direct transmission of increased blood pressure to the glomerulus increases injury. Other mechanisms may also play a role, however, since antihypertensive drugs have benefit even in nonhypertensive patients with chronic kidney disease (see below). Recent studies point to strong genetic factors linked to risk of hypertension-associated kidney injury, although mechanisms are not yet elucidated.
Pathologic Findings
Gross Findings/Light Microscopy
“Benign” nephrosclerosis results in small kidneys with finely granular surface and thinned cortex in late stages. Malignant (accelerated) nephrosclerosis grossly shows petechial hemorrhage of the subcapsular surface, with mottling and occasional areas of infarct. Microscopically, in “benign” arterionephrosclerosis there is vascular wall medial thickening with frequent afferent arteriolar hyaline deposits and varying degree of intimal fibrosis. The hyalinization is due to endothelial injury and increased pressure, leading to an insudate of plasma macromolecules. There are associated focal glomerular ischemic changes with variable thickening and wrinkling of the basement membrane and/or global sclerosis, tubular atrophy, and interstitial fibrosis (Fig. 10.1). Global sclerosis more commonly is of the obsolescent type, with fibrous material obliterating Bowman’s space. Solidified glomeruli, where the tuft is globally sclerosed without collagen in Bowman’s space, has been called “decompensated” arterionephrosclerosis. Secondary focal segmental glomerulosclerosis (FSGS) may also occur, often with associated glomerular basement membrane (GBM) corrugation and filling of Bowman’s space with fibrous material [4–10]. These morphologic features hint that the segmental sclerotic process is secondary to hypertension-associated injury, rather than idiopathic FSGS. The lesions associated with accelerated hypertension consist of mucoid change of the arterioles, often with red blood cell (RBC) fragments within the wall. In malignant hypertension, arterioles show fibrinoid necrosis, and interlobular arteries have a concentric onion-skin pattern of intimal proliferation and fibrosis, overlapping with the appearance of scleroderma and chronic thrombotic microangiopathy (Fig. 10.2) (see below). There is proportional tubulointerstitial fibrosis in arterionephrosclerosis.
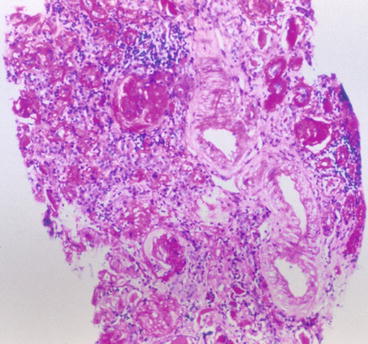
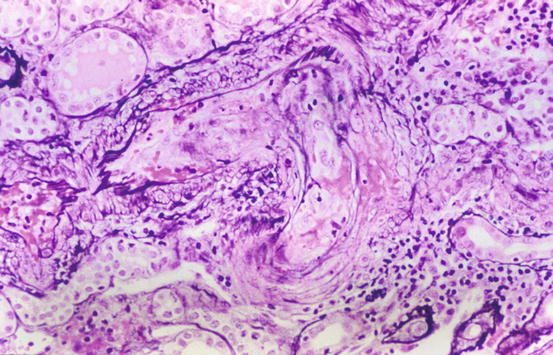

Fig. 10.1
Arterial and arteriolar medial thickening, intimal and interstitial fibrosis, tubular atrophy, and global sclerosis in arterionephrosclerosis (PAS)

Fig. 10.2
Vascular fibrinoid necrosis and thrombosis in malignant hypertension (Jones silver stain)
Immunofluorescence may show trapping of IgM and C3 in glomeruli, but there are no immune complex-type deposits. In malignant hypertension, fibrin/fibrinogen staining may be present in necrosed arterioles/arteries and injured glomeruli.
Electron microscopy confirms the corrugated, wrinkled GBM and ischemic changes with increased lamina rara interna but without immune deposits. Hyaline may be present in sclerosed segments. Some foot process effacement of podocytes may also be present, but it is usually not extensive.
Although none of the above lesions are pathognomonic, the constellation of these changes in the absence of other lesions of primary glomerular disease is indicative of arterionephrosclerosis.
Etiology/Pathogenesis
Hypertension has been presumed to cause end-organ damage in the kidney, and hypertension undoubtedly accelerates progressive scarring of renal parenchyma, but the relationship of hypertension and arterionephrosclerosis is not simple and linear [11]. In a large series of renal biopsies in patients with essential hypertension, arterionephrosclerosis was present in the vast majority, and the severity of arteriolar sclerosis correlated significantly with level of diastolic blood pressure [9]. However, in several large autopsy series of patients with presumed benign hypertension, significant renal lesions were rare [4, 5]. Further, the level of blood pressure does not directly predict degree of end-organ damage: African-Americans have higher risk for more severe end-organ damage at any level of blood pressure [2]. The African American Study of Kidney Disease (AASK) trial showed that African-Americans with presumed arterionephrosclerosis indeed did not have other lesions, by renal biopsy, but the global sclerosis was severe and did not correlate with vascular sclerosis [12]. It is possible that underlying microvascular disease causes the hypertension and the renal disease in susceptible patients. In a large study of patients without clinically evident kidney disease at baseline, even relatively modest elevation in blood pressure was an independent risk factor for development of end-stage kidney disease [13]. Underlying causes in addition to direct hemodynamic injury could include possible genetic and structural components, such as decreased nephron number and consequently fewer, but enlarged glomeruli [14]. Whether hypertension can cause kidney scarring, or a primary microvascular renal injury causes the hypertension, which in turn accelerates the sclerosis, has not been proven. Apolipoprotein L1 allele variants are tightly linked to excess arterionephrosclerosis, focal segmental glomerulosclerosis, and HIV-associated nephropathy, but not diabetic nephropathy in African-Americans [15]. The ApoL1 allele variant confers protection against some trypanosomes, which could have a survival advantage and thus, by natural selection, have led to its high prevalence in African-Americans. The mechanisms of increased risk of kidney disease are unknown [16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


