, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown cause that can occur at almost any age, although it affects mostly women in their 20s. The annual incidence of SLE is 50–70 people per million of the population, and prevalence is 500 per million [1]. The incidence of new cases and the survival of patients with SLE are both increasing [2]. The disease is characterized by a large variety of organ disorders involving many different immune mechanisms. The spectrum of kidney lesions predominantly involves the glomerulus and includes minimal mesangial alterations to florid proliferative lesions with necrosis and crescents but also extends to nonimmune complex lesions such as thrombotic microangiopathy (see Chap. 11) and direct podocyte injury. Correspondingly, clinical manifestations and course are equally diverse. Kidney disease develops in more than half of lupus patients and represents the first clinical manifestation of SLE in 15–20 % [3, 4]. Moreover, renal alterations are found in almost 90 % of lupus patients at autopsy. The lowest 5-year survival has been reported for patients with central nervous system and renal involvement [1].
Introduction/Clinical Setting
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown cause that can occur at almost any age, although it affects mostly women in their 20s. The annual incidence of SLE is 50–70 people per million of the population, and prevalence is 500 per million [1]. The incidence of new cases and the survival of patients with SLE are both increasing [2]. The disease is characterized by a large variety of organ disorders involving many different immune mechanisms. The spectrum of kidney lesions predominantly involves the glomerulus and includes minimal mesangial alterations to florid proliferative lesions with necrosis and crescents but also extends to nonimmune complex lesions such as thrombotic microangiopathy (see Chap. 11) and direct podocyte injury. Correspondingly, clinical manifestations and course are equally diverse. Kidney disease develops in more than half of lupus patients and represents the first clinical manifestation of SLE in 15–20 % [3, 4]. Moreover, renal alterations are found in almost 90 % of lupus patients at autopsy. The lowest 5-year survival has been reported for patients with central nervous system and renal involvement [1].
The diagnosis of SLE is based on the documentation of multisystem involvement that meets at least 4 of 11 criteria established by the American College of Rheumatology [5]. Lupus nephritis is typically manifest by proteinuria, ranging from minimal to nephrotic and usually correlating with the histologic type of lesion. Severe glomerular lesions cause hematuria, a telescoped urinary sediment (i.e., red and white blood cells, as well as hyaline, granular, cellular, and broad casts), and renal insufficiency. Hypertension usually develops later in the course of the disease.
Classification of the renal pathology of lupus patients has been based on light microscopic changes, combined with immunohistochemical/immunofluorescent and ultrastructural observations. The classification was most recently revised in 2004 by a working group under the auspices of the Renal Pathology Society (RPS) and the International Society of Nephrology (ISN) (Tables 8.1, 8.2, and 8.3) [6].
Table 8.1
International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of lupus nephritis [6]
Class I: minimal mesangial lupus nephritis |
Normal glomeruli by light microscopy (LM) but mesangial immune deposits by immunofluorescence (IF) and/or electron microscopy (EM) |
Class II: mesangial proliferative lupus nephritis |
Mesangial hypercellularity or mesangial matrix expansion by LM with mesangial immune deposits; a few isolated subepithelial and/or subendothelial deposits may be present |
Class III: focal lupus nephritisa |
Active (A) and/or inactive chronic (C) focal, segmental, or global endocapillary or extracapillary glomerulonephritis involving <50 % of all glomeruli |
Class IV: diffuse lupus nephritisb |
Active (A) or inactive chronic (C) diffuse, segmental (involving less than half of the glomerular tuft), or global endocapillary or extracapillary glomerulonephritis involving ≥50 % of all glomeruli |
This class is divided into diffuse segmental (IV-S) lupus nephritis when ≥50 % of the involved glomeruli have segmental lesions and diffuse global (IV-G) lupus nephritis when ≥50 % of the involved glomeruli have global lesions |
This class includes cases with diffuse wire-loop deposits but with little or no glomerular proliferation |
Class V: membranous lupus nephritis |
Subepithelial immune deposits or their morphologic sequelae by LM and by IF or EM, involving ≥50 % of glomeruli and ≥50 % of capillary loops, with or without mesangial alterations |
Class V lupus nephritis may occur in combination with class III or IV, in which case both will be diagnosed |
Class VI: advanced sclerosing lupus nephritis |
>90 % of glomeruli globally sclerosed without residual activity |
Table 8.2
Abbreviated ISN/RPS classification of lupus nephritis
Class I | Minimal mesangial lupus nephritis |
Class II | Mesangial proliferative lupus nephritis |
Class III | Focal lupus nephritisa |
Class IV | Diffuse segmental (IV-S) or global (IV-G) lupus nephritisb |
Class V | Membranous lupus nephritisc |
Class VI | Advanced sclerosing lupus nephritis |
Table 8.3
Active and chronic glomerular lesions in lupus nephritis
Active lesions |
Endocapillary hypercellularity with or without leukocyte infiltration and with substantial luminal reduction |
Karyorrhexis |
Fibrinoid necrosis |
Rupture of glomerular basement membrane |
Crescents, cellular or fibrocellular |
Subendothelial deposits identifiable by LM (wire loops) |
Intraluminal immune aggregates (hyaline thrombi) |
Chronic lesions |
Glomerular sclerosis (segmental or global) |
Fibrous adhesions |
Fibrous crescents |
Pathologic Findings
Light Microscopy, Immunofluorescence, and Electron Microscopy
Mesangial Lupus Nephritis Classes I and II
Classes I and II lupus nephritis refer to mesangial lupus nephritis. These patients present clinically with mild hematuria, or proteinuria, or both. In general, this kidney lesion has a good prognosis, and the histologic alterations remain stable in the majority of cases. However, functional deterioration and progression of glomerular lesions to more active or generalized proliferative forms occur in about 20 % of cases. In the past decades, the availability of better supportive therapy and more selective use of immunosuppressive agents have led to improved survival of patients with mild forms of lupus glomerulonephritis, while new forms of immunosuppressive therapy are being developed [7, 8].
Class I minimal mesangial lupus nephritis refers to biopsies showing normal glomeruli by light microscopy but mesangial immune deposits by immunofluorescence and/or electron microscopy. Class II contains mesangial proliferative lesions characterized by mesangial hypercellularity of any degree or mesangial matrix expansion by light microscopy with mesangial deposits. In either class I or II mesangial forms of LN, there may be a few isolated subepithelial or subendothelial deposits by immunofluorescence and/or electron microscopy but without endocapillary, sclerotic, or crescentic reactions (Fig. 8.1). If the latter changes are present, then a diagnosis of focal LN (if less than half of glomeruli manifest these reactions) or diffuse LN (more than half) is warranted.
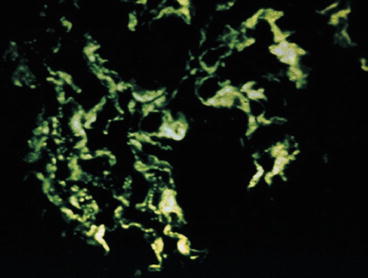

Fig. 8.1
Mesangial proliferative lupus nephritis ISN/RPS class II with granular mesangial immunoglobulin G (IgG) (immunofluorescence)
Focal Lupus Nephritis Class III
Focal lupus nephritis ISN/RPS class III entails focal (involving less than half of the glomeruli available for inspection) proliferative, necrotizing, or sclerosing lesions. These lesions may be either segmental (involving <50 % of the tuft area of the affected glomeruli) or global (involving ≥50 % of the tuft area of the involved glomeruli). A subdivision is made according to the predominance of active versus sclerotic lesions as indicated in Table 8.1.
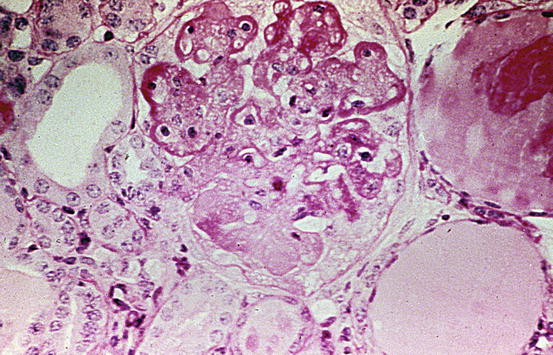
In the pathology report, the proportion of glomeruli with active and with sclerotic lesions and the proportion of glomeruli with fibrinoid necrosis or cellular crescents should be indicated. The proliferative lesions include variable mesangial proliferation and endocapillary proliferation with variable inflammatory cells. Double contours of the GBM on silver stain may be present. Necrotizing lesions with fibrinoid necrosis and crescent reaction are often present in active lesions (Figs. 8.2 and 8.3). In these areas, there is nuclear debris as well as influx of inflammatory cells. The inflammatory process may also lead to disruption of the glomerular basement membrane and fibrinoid necrosis. Fibrinoid necrosis appears as amorphous eosinophilic material staining bright red in trichrome staining, often associated with GBM breaks and karyorrhexis. Interstitial inflammation may be marked adjacent to glomeruli disrupted by crescents and/or necrosis, particularly if Bowman’s capsule is ruptured by the destructive lesion. Segmental sclerotic scars with broad-based adhesions to Bowman’s capsule can develop from focal crescents with necrotic lesions. Tubular atrophy and interstitial fibrosis are proportional to glomerular scarring.
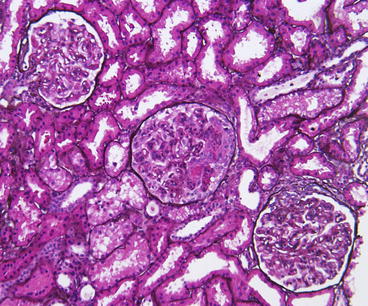
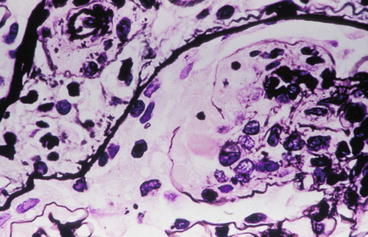

Fig. 8.2
Focal lupus nephritis ISN/RPS class III (A + C) with active endocapillary proliferation and an early necrotizing lesion with rupture of the glomerular basement membrane and early crescent formation in the middle glomerulus and a small adhesion and fibrocellular crescent in the lower right glomerulus. However, less than half of the glomeruli showed such lesions (Jones silver stain)

Fig. 8.3
Focal lupus nephritis ISN/RPS class III (A) with active endocapillary proliferation and an early necrotizing lesion with rupture of the glomerular basement membrane (Jones silver stain)
Immunofluorescent staining shows the presence of immunoglobulin G (IgG), IgM, IgA, and complement factors C3 and C1q (“full-house” immunofluorescence) in chunky granular and globular depositions along the glomerular capillary walls and in the mesangium (Fig. 8.4). These capillary wall deposits are largely subendothelial, seen by their smooth outer contour as they are molded under the GBM, and confirmed by electron microscopy. Although the light microscopic proliferative changes are focal, the immunofluorescence is usually positive in all glomeruli. Electron microscopy typically demonstrates deposits in the mesangium and also in the subendothelial area. There may be scattered subepithelial deposits, but if these are extensive (i.e., >50 % of loops in most glomeruli), membranous LN should be diagnosed in addition to the proliferative process (Fig. 8.5) [6].
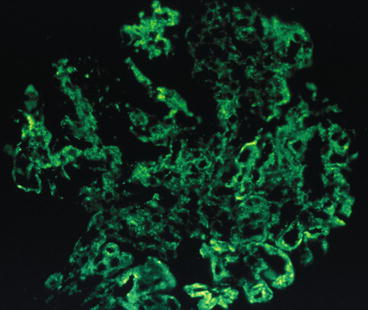
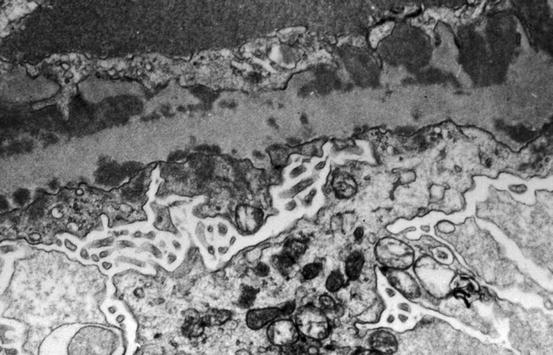

Fig. 8.4
Focal lupus nephritis ISN/RPS class III with diffuse, chunky pattern for IgG in mesangium and along capillary wall. Some of the capillary wall deposits have a smooth outer contour, reflecting their subendothelial location (immunofluorescence)

Fig. 8.5
Subendothelial (below the glomerular basement membrane, GBM) and subepithelial (above the GBM) electron dense deposits with irregular thickening of GBM and microvillous transformation of podocytes in focal lupus nephritis ISN/RPS class III (electron microscopy)
Patients with focal lupus nephritis class III present almost invariably with proteinuria and in the majority of cases with mixed findings of nephrotic and nephritic syndromes. The lesions can transform to diffuse proliferative (class IV) or membranous lupus nephritis class V.
Diffuse Lupus Nephritis Class IV
Patients with diffuse lupus nephritis class IV typically have increased renal dysfunction and significant proteinuria and active urine sediment. This class is the most common and severe form of lupus nephritis detected in renal biopsies. The biopsy shows diffuse segmental (IV-S) or global (IV-G) lesions, characterized by proliferative, sclerosing, and/or necrotizing lesions in more than 50 % of the glomeruli. The lesions may thus be either active or inactive and have a segmental or global distribution. There is variable mesangial and endocapillary proliferation. Cellular crescents and necrosis are frequently present in active cases, whereas broad-based adhesions with segmental sclerosis and fibrocellular to fibrous crescents characterize the chronic lesions. Some investigators revealed a poor outcome of diffuse “segmental” necrotizing glomerulonephritis involving over 50 % of glomeruli, as compared to other forms of class IV lupus nephritis [9]. Attempts to capture the possible significance of these severe segmental lesions were therefore made by dividing class IV diffuse lupus nephritis into diffuse segmental (IV-S) when ≥50 % of the involved glomeruli have segmental lesions and diffuse global (IV-G) when ≥50 % of the involved glomeruli have global lesions (Fig. 8.6) [6]. Furthermore, a subdivision is made according to the presence of active versus chronic lesions as was indicated for class III lesions (Table 8.1). However, the definition of “segmental” as <50 % of the tuft is not congruent with that used by the original investigators, who considered lesions “segmental” if even a single loop of the glomerular tuft was not involved. Thus, the ISN/RPS S versus G lesions may not adequately capture this subgroup of patients.
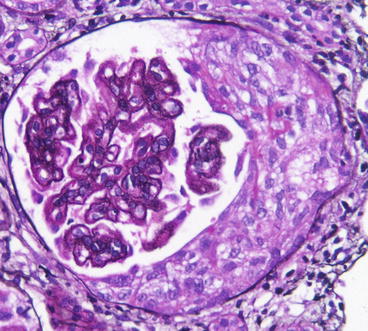

Fig. 8.6
Diffuse proliferative lupus nephritis ISN/RPS class IV with cellular crescent and segmental endocapillary proliferation and double contours of GBM (Jones silver stain)
As in focal LN class III, the proportion of glomeruli with active and with sclerotic lesions and the proportion of glomeruli with fibrinoid necrosis or cellular crescents should be indicated in the pathology report. “Wire–loop” lesions, that is, local periodic acid-Schiff (PAS)-positive thickenings of the glomerular capillary walls, are characteristic of this form of lupus nephritis (Fig. 8.7). This thickening of the capillary walls is related to the presence of large, subendothelial electron dense deposits. Glomerular lesions run the gamut from diffuse hypercellularity to severe necrotizing “crescentic” glomerulonephritis or, in chronic cases, diffuse global glomerulosclerosis with loss of renal function. Tubular atrophy and interstitial fibrosis are often more extensive in diffuse LN, class IV than in focal LN, class III. The predictive value of these lesions with respect to renal function, however, is disputed [10, 11]. The tubular epithelium shows cytoplasmic hyaline droplets, hydropic degeneration, cytoplasmic vacuolization, hyaline protein cylinders, and, in more advanced stages, disease glomerulosclerosis, tubular atrophy, and interstitial fibrosis. Arteries and arterioles may show varying lesions (see below).