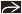Neoadjuvant Therapy
TERMINOLOGY
Abbreviations
Neoadjuvant therapy (NAT)
Synonyms
Preoperative therapy
Presurgical therapy
Definitions
NAT is treatment of patients with chemotherapy &/or hormonal therapy prior to surgical removal of carcinoma
Diagnosis of primary carcinoma is generally made by core needle biopsy; histologic type, grade, and tumor markers are determined on this specimen
Size and extent of primary carcinoma (i.e., multicentricity, involvement of skin or chest wall) is determined by imaging studies
Lymph nodes must be evaluated prior to treatment in order to gain maximum amount of information about tumor response
Palpable or enlarged nodes on ultrasound should be sampled by fine needle aspiration
If no nodes suitable for fine needle aspiration can be identified, sentinel node biopsy may be performed
However, if the only positive node is excised, response to treatment in lymph nodes cannot be determined
CLINICAL IMPLICATIONS
Response to NAT
Degree of response to NAT provides additional information
Individual patients: Prognostic information can be used to guide further treatment and help determine benefit of prophylactic surgery
Clinical trials: Response to NAT is short-term endpoint that can be used to compare different treatments using fewer patients over shorter period of time
Research: Paired samples of cancers pre- and post-treatment are valuable resource for research
Can be used to investigate types of carcinomas that respond to specific treatments
Can be used to investigate how carcinomas respond and how to predict which carcinomas will respond
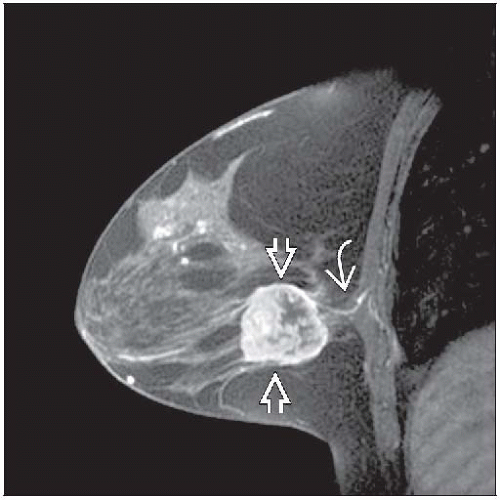
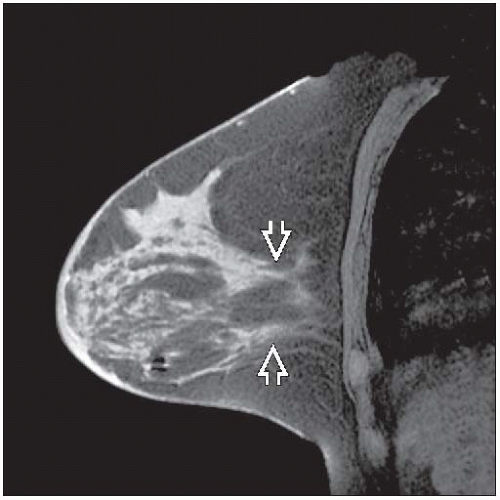
Clinical/radiologic evaluation of response may be inaccurate
Residual palpable masses and radiologic densities may be due to tumor bed without viable tumor
Carcinomas undergoing partial response typically become softer, show less uptake by MR, and break up into multiple small tumor foci
Considerable residual carcinoma can be present even if no palpable mass remains and imaging findings resolve after treatment
Pathologic evaluation of response to NAT
All prognostic factors prior to treatment are also prognostic after NAT
Additional prognostic factors after NAT have been used in multiple different systems for evaluating response
Change in tumor size
Change in tumor cellularity: Most carcinomas become less cellular
Response in lymph nodes: Response of metastatic carcinoma has greater prognostic value than response in breast
Pathologic complete response (pCR) is associated with exceptionally good outcome for majority of patients
Clinical Value of NAT
NAT does not improve survival over adjuvant therapy (treatment after surgical removal of cancer)
In some patients, decrease in size of large cancer will make them eligible for breast conservation
However, there is small increased risk of local recurrence
Primary value of NAT is prognostic and scientific information gained from degree of response of cancer to treatment
Predicting Response to NAT
Likelihood of response to therapy can be predicted by features of pre-treatment carcinoma
Response to endocrine therapy
ER status: ER/PR-positive carcinomas respond more than ER-negative carcinomas or PR-negative carcinomas
Response to chemotherapy
Grade 3 (poorly differentiated) carcinomas: More likely to respond
High mitotic rate: More likely to respond
Tumor necrosis: More likely to respond
ER negative: More likely to respond
HER2 overexpression: More likely to respond to HER2-based therapy
Lobular type: Less likely to respond
Future predictors
NAT trials are designed to develop new methods (such as RNA profiling) that will provide better indicators of response to enable more individualized treatment for patients
MACROSCOPIC FINDINGS
Specimen Radiographs
Clip should be placed prior to treatment to identify tumor bed
If there is complete or near complete response, there may not be alternative method to identify site of carcinoma
Calcifications associated with carcinoma generally remain after treatment
Not all carcinomas are associated with calcifications, and this finding should not be relied upon to identify tumor site
Specimen Handling
Tissue adjacent to clip(s) must be identified grossly
If grossly evident invasive carcinoma is present, size, number of cancers, and relationship of cancers to each other should be described
Typical partial response to treatment is seen as multiple foci of invasion scattered over tumor bed
Extent of sampling adjacent to clip(s) if gross carcinoma is not present depends on extent of carcinoma prior to treatment
If practical, entire tumor bed should be sampled if patient may have had a pCR
If tumor bed is quite large, at least 1 block of tissue per cm of tumor bed is suggested
Additional tissue sampling can be determined after examination of initial sampling
Gross presence of tumor bed at margins should be noted
All margins should be sampled with perpendicular sections
Lymph nodes should be carefully searched for and completely submitted for microscopic examination
Nodes may be smaller and fewer in number after treatment
MICROSCOPIC FINDINGS
General Features
Tumor bed must be identified microscopically
Typical findings are dense fibrosis, histiocytes, lymphocytes, occasional giant cells, and hemosiderin-laden macrophages
Tumor bed will not look like normal breast tissue
If only normal breast tissue is present, additional gross sampling is indicated
Residual invasive carcinoma usually resembles pre-treatment carcinoma but is often less cellular
In rare cases, the carcinoma may appear to be more poorly differentiated or very rarely more well differentiated
Residual DCIS may be present
In some cases, there can be pCR for invasive carcinoma but with residual DCIS
DCIS may be similar in appearance or may have higher nuclear grade
Presence of DCIS can be helpful in identifying the location of tumor bed
Normal breast tissue may undergo treatment-related changes
Ducts and lobules may appear atrophic with thickened basement membranes
Scattered cells with nuclear pleomorphism may be seen
In some cases, it may be difficult to distinguish treatment-related changes from residual DCIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree