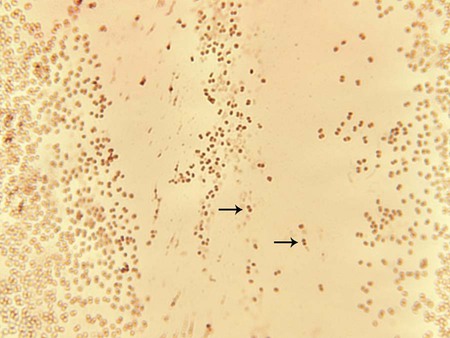
1. Identify the clinical specimens or sources for the isolation of pathogenic Neisseria spp. 2. List the Neisseria species considered normal flora and the sites where they colonize the human body. 3. Explain the routes of transmission for the organisms discussed in this chapter; include the clinical relevance of asymptomatic carriers. 4. Define and describe the diseases associated with Moraxella catarrhalis and the pathogenic Neisseria spp., Neisseria gonorrhoeae and Neisseria meningitidis (i.e., pelvic inflammatory disease, disseminated gonococcal infection, ophthalmia neonatorum, pharyngitis, meningitis, and septicemia); include the signs and symptoms, treatments, and prognosis. 5. Describe the method of transport that yields optimal recovery of N. gonorrhoeae, including transport media, growth temperatures, and atmospheric conditions. 6. Describe the benefits of amplified testing for N. gonorrhoeae over nonamplified testing as it relates to financial, diagnostic and clinical efficacy, and control measures. 7. Identify the optimal growth conditions for the Neisseria species. 8. Name the appropriate biochemical tests for differentiating the Neisseria species and explain the chemical principle for each test. 9. Biochemically differentiate the organisms in this chapter using carbohydrate utilization (cysteine trypticase agar [CTA]) and orthonitrophenyl galactoside (ONPG). 10. Describe the appropriate therapeutic agents for N. gonorrhoeae. 11. Compare and contrast the laboratory identification of M. catarrhalis and Neisseria spp. 12. Analyze laboratory data and disease signs and symptoms for correlation and identification of the etiologic agents discussed in this chapter. Species of the family Neisseriaceae, genus Neisseria, are discussed in this chapter, along with family Moraxellaceae, species Moraxella catarrhalis, because of their biochemical and morphologic similarities. The organisms are all oxidase-positive, gram-negative diplococci that do not elongate when exposed to subinhibitory concentrations of penicillin. The rodlike Neisseria spp. are described in Chapter 28. Except for Neisseria gonorrhoeae, the organisms considered in Table 40-1 are normal inhabitants of the upper respiratory tract of humans. Humans are the only natural host for N. gonorrhoeae, primarily a clinically significant pathogen found in the urogenital tract and never considered normal flora. Asymptomatic carriers of gonorrhea are the primary reservoir for dissemination in the human population. The number of reported and identified cases of infection with N. gonorrhoeae is probably significantly higher than statistical data indicate because of the number of unreported cases. TABLE 40-1 As noted in Table 40-2, infections caused by M. catarrhalis are usually localized to the respiratory tract and rarely disseminate. TABLE 40-2 Pathogenesis and Spectrum of Disease N. gonorrhoeae is a leading cause of sexually transmitted disease, and infections caused by this organism usually are localized to the mucosal surfaces where the host is initially exposed to the organism (e.g., cervix, conjunctiva, pharyngeal surface, anorectal area, or urethra of males). Localized infections may be asymptomatic or acute with a pronounced purulent response. Not all infections remain localized, and dissemination from the initial infection site can lead to severe disseminated disease (see Table 40-2). Isolates with nutritional requirements for arginine, hypoxanthine, and uracil (AHU strains) are often isolated from disseminated infections, most often from women although also from asymptomatic males. N. meningitidis is a leading cause of fatal bacterial meningitis. However, the virulence factors responsible for the spread of this organism from a patient’s upper respiratory tract to the bloodstream and meninges, causing life-threatening infections, are not fully understood (see Table 40-2). The pathogenic Neisseria spp. described in this chapter are very sensitive to drying and temperature extremes. In addition to general information on specimen collection and transport provided in Table 5-1, there are some special requirements for isolation of N. gonorrhoeae and N. meningitidis. Swabs are acceptable for N. gonorrhoeae testing if the specimen will be plated within 6 hours; however, reduced recovery may result within 30 minutes of collection. If cotton swabs are used, the transport medium should contain charcoal (Ames medium) to inhibit toxic fatty acids present in the fibers. Calcium alginate has also been found to be inhibitory. Dacron or rayon fibers are recommended. N. gonorrhoeae should be inoculated to growth media immediately after specimen collection. The sample should then be placed in a container able to sustain an atmosphere of increased carbon dioxide (CO2) during transport. Specially packaged media consisting of selective agar in plastic trays that contain a CO2-generating system are commercially available (JEMBEC plates). The JEMBEC system (Figure 40-1) is transported to the laboratory at room temperature. Upon receipt in the laboratory, the agar surface is cross-streaked to obtain isolated colonies, and the plate is incubated at 35°C in 3% to 5% CO2. Additional commercial transport systems that may be useful, particularly when the collection site is separate from the diagnostic laboratory, are the Bio-Bag, Gono-Pak, and Transgrow. The members of the genus Neisseria discussed in this chapter and M. catarrhalis appear as gram-negative diplococci (Figure 40-2) with adjacent sides flattened. They are often referred to as “kidney bean”–shaped diplococci. Direct Gram staining of urethral discharge from symptomatic males with urethritis is an important test for gonococcal disease. The appearance of gram-negative diplococci inside polymorphonuclear leukocytes is diagnostic in this situation. However, because the normal vaginal and rectal flora are composed of gram-negative coccobacilli, which can resemble Neisseria spp., direct examination of endocervical secretions in symptomatic women is only presumptive evidence of gonorrhea, and the diagnosis must be confirmed by culture. In addition, avirulent strains (i.e., pili types 3 to 5) may be present as extracellular diplococci; these are not pathogenic. Pharyngeal specimens should not be Gram stained, because nonpathogenic, commensal Neisseria spp. may be present, and these are not diagnostic of infection. Molecular assays have replaced old enzyme-linked immunosorbent assay systems for rapid diagnosis of N. gonorrhoeae. The U.S. Food and Drug Administration (FDA) has cleared a number of amplified and nonamplified tests. (For a discussion of molecular technology, see Chapter 8.) The nonamplified DNA probe assay, PACE 2 (Hologic, Inc., Bedford, MA), has a chemiluminescent detection system for direct detection of gonococcal ribosomal RNA (rRNA) in genital and conjunctival specimens. This test performs well in high-risk patients, is rapid (results are available in 2 hours), and is suitable for screening many patients simultaneously. The Gen-Probe Accuprobe test targets rRNA after lysis of bacteria; the rRNA is detected using a single-strand chemiluminescent DNA probe. The hybrids are then detected in a luminometer. In addition, the Digene CT/GC Dual ID HC2 (HC2; Qiagen) detects RNA-DNA hybrids using antibody-mediated recognition of the hybrids and visualization of a chemiluminescent substrate. New York City (NYC) medium, a transparent medium containing lysed horse blood, horse plasma, yeast dialysate, and the same antibiotics as MTM, also has been used. The advantage of NYC medium is that genital mycoplasmas (Mycoplasma hominis and Ureaplasma urealyticum; see Chapter 45) also grow on this agar. Some strains of N. gonorrhoeae are inhibited by the concentration of vancomycin in the selective media, so the addition of nonselective chocolate agar is recommended, especially in suspect cases that are culture negative or for sterile specimens (e.g., joint fluid). Humidity can be provided by placing a pan with water in the bottom of a CO2 incubator or by placing a sterile gauze pad soaked with sterile water in the bottom of a candle jar (Figure 40-3).
Neisseria and Moraxella catarrhalis
General Characteristics
Epidemiology
Organism
Habitat (Reservoir)
Mode of Transmission
Moraxella catarrhalis
Normal human flora of upper respiratory tract; occasionally colonizes female genital tract
Spread of patient’s endogenous strain to normally sterile sites. Person-to-person nosocomial spread by contaminated respiratory droplets also can occur
Neisseria gonorrhoeae
Not part of normal human flora. Only found on mucous membranes of genitalia, anorectal area, oropharynx, or conjunctiva at time of infection
Person-to-person spread by sexual contact, including rectal intercourse and orogenital sex. May also be spread from infected mother to newborn during birth.
Asymptomatic carriers are a significant reservoir for increased disease transmission.
Neisseria meningitidis
Colonizes oropharyngeal and nasopharyngeal mucous membranes of humans. Humans commonly carry the organism without symptoms
Person-to-person spread by contaminated respiratory droplets, usually in settings of close contact
Other Neisseria spp.
Normal human flora of the upper respiratory tract
Spread of patient’s endogenous strain to normally sterile sites. Person-to-person spread may also be possible, but these species are not common causes of human infections
Neisseria animaloris
Not part of normal human flora. Animal oral and respiratory commensal organism
Animal contact, particularly bites or scratches from dogs and cats
Pathogenesis and Spectrum of Disease
Organism
Virulence Factors
Spectrum of Disease and Infections
Moraxella catarrhalis
Uncertain; factors associated with cell envelope probably facilitate attachment to respiratory epithelial cells
Most infections are localized to sites associated with the respiratory tract and include otitis media, sinusitis, and pneumonia. Lower respiratory tract infections often target elderly patients and those with chronic obstructive pulmonary disease. Rarely causes disseminated infections such as bacteremia or meningitis.
Neisseria gonorrhoeae
Several surface factors, such as pili (types T1-T2 virulent and T3-T5 avirulent), mediate the exchange of genetic material between strains and attachment to human mucosal cell surface, invasion of host cells, and survival through the inhibition of phagocytosis in the presence neutrophils.
Genetic-phase variation of pilus structure between types T1 through T5 allows the organism to vary its antigenic structure, preventing recognition by host immune cells.
Capsule, lipooligosaccharide (endotoxin), and outer cell membrane proteins I-III are important in antigenic variation and for eliciting an inflammatory response.
Protein II (Opa) facilitates adherence to phagocytic and epithelial cells.
Protein II (RMP) blocks the bactericidal effect of host IgG.
Outer membrane porin (PorB) provides protection from the host’s immune response, including serum complement–mediated cell death.
A leading cause of sexually transmitted diseases. Genital infections include acute purulent urethritis, prostatitis, and epididymitis in males and acute cervicitis in females. These infections also may be asymptomatic in females.
Other localized infections include pharyngitis, anorectal infections, and conjunctivitis (e.g., ophthalmia neonatorum of newborns acquired during birth from an infected mother).
Disseminated infections result when the organism spreads from a local infection to cause pelvic inflammatory disease or disseminated gonococcal infection that includes bacteremia, arthritis, and metastatic infection at other body sites.
Pelvic inflammatory disease (PID) may cause sterility, ectopic pregnancy or perihepatitis also referred to as Fitz-Hugh–Curtis syndrome.
Neisseria meningitidis
Surface structures, perhaps pili, facilitate attachment to mucosal epithelial cells and invasion to the submucosa. Once in the blood, survival is mediated by production of a polysaccharide capsule. Endotoxin release mediates many of the systemic manifestations of infection, such as shock.
Cellular proteins are similar to those described for N. gonorrhoeae, including Por and Opa. Two porin proteins are produced (PorA and PorB).
IgA protease degrades membrane-associated IgA, increasing the host’s susceptibility to invasion.
Life-threatening, acute, purulent meningitis. Meningitis may be accompanied by appearance of petechiae (i.e., rash) that is associated with meningococcal bacteremia (i.e., meningococcemia). Bacteremia leads to thrombocytopenia, disseminated intravascular coagulation, and shock. Disseminated disease is often fatal. Less common infections include conjunctivitis, pneumonia, and sinusitis.
Other Neisseria spp.
Unknown; probably of low virulence
Rarely involved in human infections. When infections occur, they can include bacteremia, endocarditis, and meningitis.
Neisseria animaloris
Unknown
Cellulitis or abscess formation secondary to infected bite wounds; systemic infection (rare).
Laboratory Diagnosis
Specimen Collection and Transport
Direct Detection Methods
Gram Stain
Commercial Molecular Assays
Cultivation
Media of Choice
Incubation Conditions and Duration

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neisseria and Moraxella catarrhalis