Needle Core Biopsy Specimens of the Breast: Pathologic Processing, Examination, and Reporting Thereof
Syed A. Hoda
SPECIMEN FIXATION
Immediately after procurement, the needle core biopsy (NCB) specimen should be placed in 10% neutral buffered formaldehyde to preserve cytologic detail and to minimize drying and degradation of biologic markers, such as hormone receptors. Although microcalcifications are detectable in surgical biopsy specimens after immersion in buffered 10% formaldehyde for periods of 2 or more weeks, long periods of immersion of NCB specimens in fixative may result in radiographic disappearance of calcifications (1). The impact of apparent radiographic dissolution on the histologic appearance of calcifications has not been reported. Preservation of microcalcifications for up to 2 weeks has been described for specimens stored in 74.1% ethanol, with 1% 2-propanol (1).
REQUISITION FORM
The requisition form submitted with the NCB specimen should specify the following: patient identification data, including age and gender; laterality; clinical indication(s) for the procedure, including relevant history, prior biopsies, clinical diagnosis; sampled site; and specifics of the samples, such as the presence or absence of calcifications. The name of the submitting physician and date of the procedure must also be provided. The sampled site is generally indicated by a clock-face designation and distance from the nipple (e.g., right breast, 2 o’clock, N4) indicating that the specimen was taken from the upper inner quadrant of right breast from the 2 o’clock position at a site 4 cm from the center of the nipple). Multiple NCB specimens obtained from palpable as well as nonpalpable lesions are feasible and safe and may influence treatment (2). A wide variety of instruments are available to procure NCB specimens from the breast, and the instrument used in each case may be indicated. The specimen container should also be appropriately labeled with patient information that matches identifying information on the specimen requisition.
GROSS DESCRIPTION
A gross description should be recorded for each specimen documenting the number of cores, the range of
their lengths, and any other notable features (e.g., color). The entire specimen, including any blood clot, must be embedded for histologic study. The bottom of the lid of the specimen container should also be routinely examined for any tissue that may be stuck to it. If the material in a sample is too abundant to place in one tissue cassette, the cores should be separated into groups of approximately equal number and size. The number of cassettes corresponding to each sample should be recorded, and each cassette should be labeled with a unique identifier.
their lengths, and any other notable features (e.g., color). The entire specimen, including any blood clot, must be embedded for histologic study. The bottom of the lid of the specimen container should also be routinely examined for any tissue that may be stuck to it. If the material in a sample is too abundant to place in one tissue cassette, the cores should be separated into groups of approximately equal number and size. The number of cassettes corresponding to each sample should be recorded, and each cassette should be labeled with a unique identifier.
SPECIMEN PROCESSING
Routine methods of paraffin embedding, sectioning, and staining with hematoxylin and eosin (H&E) can be used for NCB specimens from the breast, and the tissue can be processed with other small surgical pathology specimens. The cores must be embedded in a manner that positions all samples at approximately the same plane in the paraffin block. Histologic sections should be 4 to 5 microns thick. Microscopic examination of at least three “levels” (interval sections) is recommended. These levels are generally taken approximately 50 microns apart but could be closer, depending on quantity of material available. The examination of a minimum of five levels has been recommended to ensure maximum sensitivity for detecting “atypical foci” (3). It is important not to exhaust the biopsy tissue in the preparation of initial histologic sections to preserve material for immunohistochemical studies that may be necessary to establish or refine a diagnosis. If laboratory resources allow, intervening sections cut between the various stained levels can be mounted unstained on labeled slides and saved for immunohistochemical studies. Such a protocol saves tissue, time, and effort that may be subsequently spent in the retrieval and processing of tissue blocks. If recuts are made at a second sitting for immunostains, one new recut slide should be submitted for H&E staining.
RADIOGRAPHIC CORRELATION
Needle core biopsy specimens derived from a lesion with calcifications, as demonstrated by mammography, should undergo specimen radiography immediately after the procedure, and the presence of calcification in the biopsy should be confirmed. This process makes it possible to identify and segregate the NCB samples containing calcifications from those without demonstrable calcifications prior to submission to the pathology laboratory. The cores with and without calcifications from each biopsy site can then be immersed in fixative in separately labeled containers. Alternatively, the two sets of cores can be placed into separate tissue cassettes, differentiated by color and/or label, and submitted in a single container. The method chosen to separate specimens prior to submission to the pathology laboratory should be standardized within a given institution. The practice of separating the specimens with and without calcifications is useful for correlation with the mammogram. However, recent studies suggest that the diagnoses are similar in both parts in the majority of cases (4,5,6). Equally careful attention should be paid to samples with and without calcifications.
Calcifications can be visualized in x-rays of paraffin blocks and remain detectable in this condition for an indefinite period. Radiographic study of the paraffin blocks may be helpful to determine the location of calcifications. However, calcifications that are less than 100 µm in maximum dimension are unlikely to be radiographically visible (7). Consequently, histologically detected calcifications of this small dimension cannot be assumed to constitute the calcifications seen in a clinical mammogram.
One possible explanation for the occasional lack of histologic visualization of calcification in NCB material obtained for mammographically detected microcalcification is their loss during histologic sectioning. This may happen either due to the discarding of shavings containing calcifications in the microtome or “fracturing” and consequent ejection of chunky calcific deposits during the preparation of levels. Indeed, radiography of histologic shavings has provided evidence for both of these rare events (8). Also it is important to be aware of the possibility that calcifications from vacuum-assisted core biopsy procedures may be aspirated into the debris canister (9). Other explanations for “missing” calcification are the lack of correlation with radiographic findings, failure to recognize calcium oxalate deposits, and inadequate sampling.
Whenever a biopsy is performed for calcifications, the pathology report should specify whether calcifications are detected histologically and the type of lesion in which they are located. If calcifications are described in the radiograph of the NCB specimens and none are initially evident histologically, the slides should be examined for birefringent calcium oxalate (weddellite) crystals. These crystals do not stain with the H&E stain but are birefringent with polarized light (10). Calcium oxalate crystals are usually located in cysts lined by apocrine epithelium, and they sometimes elicit a foreign body giant cell reaction in the stroma.
FROZEN SECTION EXAMINATION
As a rule, frozen section examination (FSE) should not be performed on NCB samples obtained from nonpalpable, radiographically detected breast lesion or on NCB samples from palpable tumors. These recommendations are based on the following observations: (a) Interpretation of diagnostically difficult lesions is compromised by frozen section artifact, increasing the risk of misdiagnosis, and (b) part of the diagnostic tissue may be wasted in the process of preparing the frozen section slide.
FSE of an NCB specimen from a palpable breast tumor might be appropriate in an emergency situation. Optimally, a request for a frozen section consultation on an NCB specimen should be discussed with the responsible pathologist preoperatively. FSE is a particularly inappropriate method for rendering a diagnosis if nonsurgical ablation of a tumor or neoadjuvant chemotherapy is being considered. It is imperative that H&E-stained paraffin sections be prepared, and deemed diagnostic, before treatments that would radically alter the remainder the lesion are undertaken.
TOUCH IMPRINT CYTOLOGY
Touch imprint cytology (TIC) of an NCB specimen is a technique that provides a cytologic diagnosis without the risks of tissue loss attendant on preparing a frozen section (11,12,13,14). TIC of a NCB specimen has been proclaimed as a “new method for same-day diagnosis” (12). However, this procedure substitutes the limitations of cytologic preparation for those of frozen sections. Imprints are subject to drying artifacts and other distortions that present substantial pitfalls for experienced and inexperienced pathologists.
TIC is prepared by either “touching” (i.e., gently compressing) or “rolling” the NCB specimen on glass slides. Air-dried slides are suitable for the Diff-Quik stain (Fig. 29.1). Alcohol-fixed slides may be used for H&E or Papanicolaou stains. TIC may be of value in the immediate assessment of specimen adequacy, thus reducing the number of insufficient specimens. As shown in Table 29.1, the sensitivity of TIC has been reported to range from 74% to 91% and specificity from 89% to 97%. The interpretation of low-grade carcinoma and fibroadenoma in a TIC preparation may be particularly challenging (14). We recommend that TIC of breast NCB specimen be undertaken only by pathologists or cytologists who see this type of specimen with sufficient frequency to maintain a high level of proficiency and who work in close collaboration with radiologists and surgeons who perform the biopsy procedure.
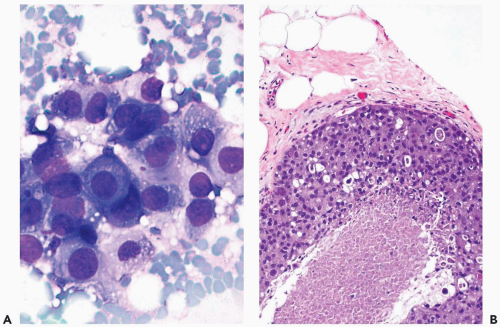 Figure 29.1 Touch imprint cytology of intraductal carcinoma. A. The touch imprint prepared
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|