Natural Products in Cancer Chemotherapy: Hormones and Related Agents
The growth of a number of cancers is hormone-dependent or regulated by hormones. Glucocorticoids are used for their antiproliferative and lympholytic properties and to ameliorate untoward responses to other treatments. Estrogen, androgen, and GnRH analogs and antagonists are effective in extending survival and delaying or preventing tumor recurrence of both breast and prostate cancer. These molecules interrupt the stimulatory axis created by systemic pools of androgens and estrogens, inhibit hormone production or hormone binding to receptors, and ultimately block expression of genes that promote tumor growth and survival.
GLUCOCORTICOIDS
The pharmacology, major therapeutic uses, and toxic effects of the glucocorticoids are discussed in Chapter 42. Only the applications of these drugs in the treatment of neoplastic disease are considered here.
Glucocorticoids act by binding to a specific physiological receptor that translocates to the nucleus and induces antiproliferative and apoptotic responses in sensitive cells. Because of their lympholytic effects and their ability to suppress mitosis in lymphocytes, glucocorticoids are used as cytotoxic agents in the treatment of acute leukemia in children and malignant lymphoma in children and adults. In acute lymphoblastic or undifferentiated leukemia of childhood, glucocorticoids may produce prompt clinical improvement and objective hematological remissions in ≤30% of children. However, the duration of remission is brief. Remissions occur more rapidly with glucocorticoids than with antimetabolites, and there is no evidence of cross-resistance to unrelated agents. Thus, therapy is initiated with prednisone and vincristine, often followed by an anthracycline or methotrexate, and L–asparaginase. Glucocorticoids are a valuable component of curative regimens for other lymphoid malignancies, including Hodgkin disease, non-Hodgkin lymphoma, multiple myeloma, and chronic lymphocytic leukemia (CLL). Glucocorticoids are extremely helpful in controlling autoimmune hemolytic anemia and thrombocytopenia associated with CLL.
A number of glucocorticoids are available and at equivalent dosages exert similar effects (see Chapter 42). Prednisone, e.g., usually is administered orally in doses as high as 60-100 mg, for the first few days and gradually reduced to levels of 20-40 mg/day, using the lowest possible effective dose. Side effects of these agents include glucose intolerance, immunosuppression, osteoporosis, and psychosis (see Chapter 42). Dexamethasone is the preferred agent for remission induction in multiple myeloma, usually in combination with melphalan, anthracyclines, vincristine, bortezomib, or thalidomide. Glucocorticoids, particularly dexamethasone, are used in conjunction with radiotherapy to reduce edema related to tumors in critical areas such as the superior mediastinum, brain, and spinal cord. Doses of 4-6 mg every 6 h have dramatic effects in restoring neurological function in patients with cerebral metastases, but these effects are temporary. Acute changes in dexamethasone dosage can lead to a rapid recrudescence of symptoms. Dexamethasone should not be discontinued abruptly in patients receiving radiotherapy or chemotherapy for brain metastases.
PROGESTINS
Progestational agents (see Chapters 40 and 66) are used as second-line hormonal therapy for metastatic hormone-dependent breast cancer and in the management of endometrial carcinoma previously treated by surgery and radiotherapy. In addition, progestins stimulate appetite and restore a sense of well-being in cachectic patients with advanced stages of cancer and AIDS.
Medroxyprogesterone (DEPO-PROVERA, others) can be administered intramuscularly in doses of 400-1000 mg weekly. An alternative oral agent is megestrol acetate (MEGACE, others; 40-320 mg daily in divided doses). Hydroxyprogesterone (not available in the U.S.) usually is administered intramuscularly in doses of 1000 mg 1 or more times weekly. Beneficial effects have been observed in one-third of patients with endometrial cancer. The response of breast cancer to megestrol is predicted by both the presence of estrogen receptors (ERs) and the evidence of response to a prior hormonal treatment. The effect of progestin therapy in breast cancer appears to be dose dependent, with some patients demonstrating second responses following escalation of megestrol to 1600 mg/day. Clinical use of progestins in breast cancer has been largely superseded by the advent of tamoxifen and the aromatase inhibitors (AIs).
ESTROGENS AND ANDROGENS
The pharmacology of the estrogens and androgens appears in Chapters 40, 41, and 66. These agents are of value in certain neoplastic diseases, notably those of the prostate and mammary gland, because these organs are dependent on hormones for their growth, function, and morphological integrity.
High doses of estrogen have long been recognized as effective treatment of breast cancer. Paradoxically, anti-estrogens also are effective. Thus, because of equivalent efficacy and more favorable side effects, anti-estrogens such as tamoxifen and the AIs have replaced estrogens or androgens for breast cancer. The presence of the ERs and progesterone receptors (PRs) on tumor tissue serves as a biomarker for response to hormonal therapy in breast cancer and identifies the subset of patients with a ≥60% likelihood of responding. The response rate to anti-estrogen treatment is somewhat lower in the subset of patients with tumors that are ER+ or PR+ but also positive for human epidermal growth factor receptors HER1/neu amplification. In contrast, ER-negative and PR-negative carcinomas do not respond to hormonal therapy. Responses to hormonal therapy may not be apparent clinically or by imaging for 8-12 weeks. The medication typically should be continued until the disease progresses or unwanted toxicities develop. The duration of an induced remission in patients with metastatic disease averages 6-12 months but sometimes can last for many years.
ANTI-ESTROGEN THERAPY
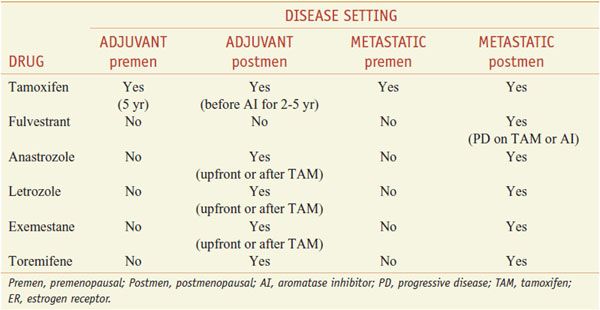
Anti-estrogen approaches for the therapy of hormone receptor–positive breast cancer include the use of selective estrogen-receptor modulators (SERMs), selective estrogen-receptor downregulators (SERDs), and AIs (Table 63–1).
Table 63–1
Clinical Uses for Anti-Estrogen Therapy in ER+ Breast Cancer
SELECTIVE ESTROGEN-RECEPTOR MODULATORS
SERMs bind to the ER and exert either estrogenic or anti-estrogenic effects, depending on the specific organ. Tamoxifen citrate is the most widely studied anti-estrogenic treatment in breast cancer. However tamoxifen also exerts estrogenic agonist effects on non-breast tissues, which influences the overall therapeutic index of the drug. Therefore, several novel anti-estrogen compounds that offer the potential for enhanced efficacy and reduced toxicity compared with tamoxifen have been developed. These novel anti-estrogens can be divided into tamoxifen analogs (e.g., toremifene [FARESTON], droloxifene, idoxifene), “fixed ring” compounds (e.g., raloxifene [EVISTA], lasofoxifene, arzoxifene, miproxifene, levormeloxifene, EM652), and the SERDs (e.g., fulvestrant [FASLODEX], SR 16234, and ZK 191703, the latter also termed “pure anti-estrogens”).
TAMOXIFEN
Tamoxifen was developed as an oral contraceptive but instead was found to induce ovulation and to have antiproliferative effects on estrogen-dependent breast cancer cell lines. Tamoxifen is prescribed for the prevention of breast cancer in high-risk patients, for the adjuvant therapy of early-stage breast cancer, and for the therapy of advanced breast cancer. It also prevents the development of breast cancer in women at high risk based on a strong family history, prior nonmalignant breast pathology, or inheritance of the BRCA1 or BRCA2 genes.
Mechanism of Action. Tamoxifen is a competitive inhibitor of estradiol binding to the ER. There are 2 subtypes of ERs: ERα and ERβ, which have different tissue distributions and can either homo- or heterodimerize. Binding of estradiol and SERMs to the estrogen-binding sites of the ERs initiates a change in conformation of the ER, dissociation of the ER from heat-shock proteins, and inhibition of ER dimerization. Dimerization facilitates the binding of the ER to specific DNA estrogen-response elements (EREs) in the vicinity of estrogen-regulated genes. Co-regulator proteins interact with the receptor to act as co-repressors or co-activators of gene expression. Differences in tissue distribution of ER subtypes, the function of co-regulator proteins, and the various transcriptional activating factors likely explain the variability of response to tamoxifen in hormone receptor–positive (ER+) breast cancer and its agonist and antagonist activities in noncancerous tissues. Other organs displaying agonist effects of tamoxifen include the uterine endometrium (endometrial hypertrophy, vaginal bleeding, and endometrial cancer), the coagulation system (thromboembolism), bone metabolism (increase in bone mineral density [BMD]), and liver (tamoxifen lowers total serum cholesterol, low-density-lipoprotein cholesterol, and lipoproteins and raises apolipoprotein A-I levels).
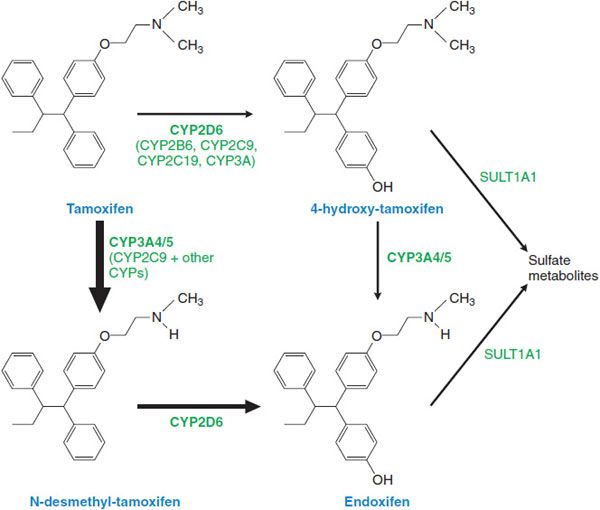
ADME. Tamoxifen is readily absorbed following oral administration, with peak concentrations measurable after 3-7 h and steady-state levels being reached at 4-6 weeks. Metabolism of tamoxifen is complex and principally involves CYPs 3A4/5, and 2D6 in the formation of N-desmethyl tamoxifen, and CYP2D6 to form 4-hydroxytamoxifen, a more potent metabolite (Figure 63–1). Both metabolites can be further converted to 4-hydroxy-N-desmethyltamoxifen, which retains high affinity for the ER. The parent drug has a terminal t1/2 of 7 days; the t1/2 of N-desmethyltamoxifen and 4-hydroxytamoxifen are significantly longer (14 days). After enterohepatic circulation, glucuronides and other metabolites are excreted in the stool; excretion in the urine is minimal.
Figure 63–1 Tamoxifen and its metabolites.
Therapeutic Uses. The usual oral dose of tamoxifen in the U.S. is 20 mg once a day. Tamoxifen is used for the endocrine treatment of women with ER+ metastatic breast cancer or following primary tumor excision as adjuvant therapy. For the adjuvant treatment of premenopausal women, tamoxifen is given for 5 years, or in postmenopausal women, for 2 years, followed by an AI. In patients with high risk of recurrence, tamoxifen may be sequenced after adjuvant chemotherapy. Tamoxifen is used in premenopausal women with ER+ tumors. Alternative or additional anti-estrogen strategies in premenopausal women include oophorectomy or gonadotropin-releasing hormone analogs. The combination of tamoxifen and a GnRH analog in premenopausal women (to reduce high estrogen levels resulting from tamoxifen effects on the gonadal-pituitary axis) yields better response rates and improved overall survival than either drug alone. Tamoxifen also has shown effectiveness (a 40-50% reduction in tumor incidence) in initial trials for preventing breast cancer in women at increased risk. Tamoxifen only reduces ER+ tumors without affecting ER-negative tumors, which contribute disproportionately to breast cancer mortality.
Toxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree