21
Mycobacteria
CHAPTER CONTENTS
INTRODUCTION
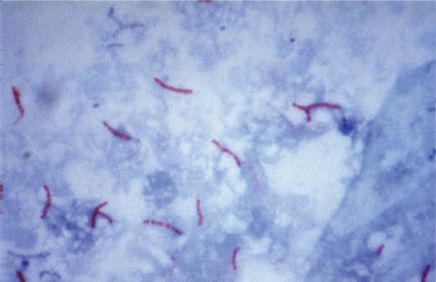
Mycobacteria are aerobic, acid-fast bacilli (rods) (Figure 21–1). They are neither gram-positive nor gram-negative (i.e., they are stained poorly by the dyes used in Gram stain). They are virtually the only bacteria that are acid-fast. (One exception is Nocardia asteroides, the major cause of nocardiosis, which is also acid-fast.) The term acid-fast refers to an organism’s ability to retain the carbolfuchsin stain despite subsequent treatment with an ethanol–hydrochloric acid mixture. The high lipid content (approximately 60%) of their cell wall makes mycobacteria acid-fast.
FIGURE 21–1 Mycobacterium tuberculosis—acid-fast stain. Long red rods of M. tuberculosis are seen on a blue background. (Figure courtesy of Dr. George Kubica, Public Health Image Library, Centers for Disease Control and Prevention.)
The major pathogens are Mycobacterium tuberculosis, the cause of tuberculosis, and Mycobacterium leprae, the cause of leprosy. Atypical mycobacteria, such as Mycobacterium avium-intracellulare complex and Mycobacterium kansasii, can cause tuberculosis-like disease but are less frequent pathogens. Rapidly growing mycobacteria, such as Mycobacterium chelonae, occasionally cause human disease in immunocompromised patients or those in whom prosthetic devices have been implanted (Table 21–1). The clinical features of three important mycobacteria are described in Table 21–2.
MYCOBACTERIUM TUBERCULOSIS
Disease
This organism causes tuberculosis. Worldwide, M. tuberculosis causes more deaths than any other single microbial agent. Approximately one-third of the world’s population is infected with this organism. Each year, it is estimated that 1.7 million people die of tuberculosis and that 9 million new cases occur. An estimated 500,000 people are infected with a multidrug-resistant strain of M. tuberculosis.
Important Properties
M. tuberculosis grows slowly (i.e., it has a doubling time of 18 hours, in contrast to most bacteria, which can double in number in 1 hour or less). Because growth is so slow, cultures of clinical specimens must be held for 6 to 8 weeks before being recorded as negative. M. tuberculosis can be cultured on bacteriologic media, whereas M. leprae cannot. Media used for its growth (e.g., Löwenstein-Jensen medium) contain complex nutrients (e.g., egg yolk) and dyes (e.g., malachite green). The dyes inhibit the unwanted normal flora present in sputum samples.
M. tuberculosis is an obligate aerobe; this explains its predilection for causing disease in highly oxygenated tissues such as the upper lobe of the lung and the kidney. The acid-fast property of M. tuberculosis (and other mycobacteria) is attributed to long-chain (C78–C90) fatty acids called mycolic acids in the cell wall.
Cord factor (trehalose dimycolate) is correlated with virulence of the organism. Virulent strains grow in a characteristic “serpentine” cordlike pattern, whereas avirulent strains do not. The organism also contains several proteins, which, when combined with waxes, elicit delayed hypersensitivity. These proteins are the antigens in the purified protein derivative (PPD) skin test (also known as the tuberculin skin test). A lipid located in the bacterial cell wall called phthiocerol dimycocerosate is required for pathogenesis in the lung.
M. tuberculosis is relatively resistant to acids and alkalis. NaOH is used to concentrate clinical specimens; it destroys unwanted bacteria, human cells, and mucus but not the organism. M. tuberculosis is resistant to dehydration and therefore survives in dried expectorated sputum; this property may be important in its transmission by aerosol.
Strains of M. tuberculosis resistant to the main antimycobacterial drug, isoniazid (isonicotinic acid hydrazide, INH), as well as strains resistant to multiple antibiotics (called multidrug-resistant or MDR strains), have become a worldwide problem. This resistance is attributed to one or more chromosomal mutations, because no plasmids have been found in this organism. One of these mutations is in a gene for mycolic acid synthesis, and another is in a gene for catalase-peroxidase, an enzyme required to activate INH within the bacterium.
Transmission & Epidemiology
M. tuberculosis is transmitted from person to person by respiratory aerosol, and its initial site of infection is the lung. In the body, it resides chiefly within reticuloendothelial cells (e.g., macrophages). Humans are the natural reservoir of M. tuberculosis. Although some animals can be infected, they are not a reservoir for human infection. Most transmission occurs by aerosols generated by the coughing of “smear-positive” people (i.e., those whose sputum contains detectable bacilli in the acid-fast stain). However, about 20% of people are infected by aerosols produced by the coughing of “smear-negative” people.
In the United States, tuberculosis is almost exclusively a human disease. In developing countries, Mycobacterium bovis also causes tuberculosis in humans. M. bovis is found in cow’s milk, which, unless pasteurized, can cause gastrointestinal tuberculosis in humans. The disease tuberculosis occurs in only a small number of infected individuals. In the United States, most cases of tuberculosis are associated with reactivation in elderly, malnourished men. The risk of infection and disease is highest among socioeconomically disadvantaged people, who have poor housing and poor nutrition. These factors, rather than genetic ones, probably account for the high rate of infection among Native Americans, African Americans, and Native Alaskans.
Pathogenesis
M. tuberculosis produces no exotoxins and does not contain endotoxin in its cell wall. In fact, no mycobacteria produce toxins. The organism preferentially infects macrophages and other reticuloendothelial cells. M. tuberculosis survives and multiplies within a cellular vacuole called a phagosome. The organism produces a protein called “exported repetitive protein” that prevents the phagosome from fusing with the lysosome, thereby allowing the organism to escape the degradative enzymes in the lysosome.
Lesions are dependent on the presence of the organism and the host response. There are two types of lesions:
(1) Exudative lesions, which consist of an acute inflammatory response and occur chiefly in the lungs at the initial site of infection.
(2) Granulomatous lesions, which consist of a central area of giant cells containing tubercle bacilli surrounded by a zone of epithelioid cells. These giant cells, called Langhans’ giant cells, are an important pathologic finding in tuberculous lesions. A tubercle is a granuloma surrounded by fibrous tissue that has undergone central caseation necrosis. Tubercles heal by fibrosis and calcification.
The primary lesion of tuberculosis usually occurs in the lungs. The parenchymal exudative lesion and the draining lymph nodes together are called a Ghon complex. Primary lesions usually occur in the lower lobes, whereas reactivation lesions usually occur in the apices. Reactivation lesions also occur in other well-oxygenated sites such as the kidneys, brain, and bone. Reactivation is seen primarily in immunocompromised or debilitated patients.
Spread of the organism within the body occurs by two mechanisms:
(1) A tubercle can erode into a bronchus, empty its caseous contents, and thereby spread the organism to other parts of the lungs, to the gastrointestinal tract if swallowed, and to other persons if expectorated.
(2) It can disseminate via the bloodstream to many internal organs. Dissemination can occur at an early stage if cell-mediated immunity fails to contain the initial infection or at a late stage if a person becomes immunocompromised.
Immunity & Hypersensitivity
After recovery from the primary infection, resistance to the organism is mediated by cellular immunity (i.e., by CD4-positive T cells and macrophages). The CD4-positive T cells are Th-1 helper T cells (see Chapter 58).
Circulating antibodies also form, but they play no role in resistance and are not used for diagnostic purposes. Patients deficient in cellular immunity, such as patients with acquired immunodeficiency syndrome (AIDS), are at much higher risk for disse minated, life-threatening tuberculosis. Mutations in the interferon-γ receptor gene are another cause of defective cellular immunity that predisposes to severe tuberculosis. This emphasizes the importance of activation of macrophages by interferon-γ in the host defense against M. tuberculosis.
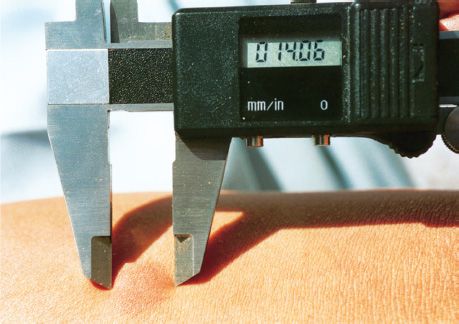
Prior infection can be detected by a positive tuberculin skin test result, which is due to a delayed hypersensitivity reaction. PPD is used as the antigen in the tuberculin skin test. The intermediate-strength preparation of PPD, which contains five tuberculin units, is usually used. The skin test is evaluated by measuring the diameter of the induration surrounding the skin test site (Figure 21–2). Note that induration (thickening), not simply erythema (reddening), must be observed.
FIGURE 21–2 Tuberculin skin test. Purified protein derivative (PPD) was injected intradermally, and 48 hours later, the diameter of induration was measured with a caliper. (Reproduced with permission from Talaro KP. Foundations in Microbiology. 8th ed. New York: McGraw-Hill, 2011.)
The diameter required to judge the test as positive varies depending on the status of the individual being tested. Induration of 15 mm or more is positive in a person who has no known risk factors. Induration of 10 mm or more is positive in a person with high-risk factors, such as a homeless person, intravenous drug users, or nursing home residents. Induration of 5 mm or more is positive in a person who has deficient cell-mediated immunity (e.g., AIDS patients) or has been in close contact with a person with active tuberculosis.
A positive skin test result indicates previous infection by the organism but not necessarily active disease. The tuberculin test becomes positive 4 to 6 weeks after infection. Immunization with bacillus Calmette-Guérin (BCG) vaccine (see page 185) can cause a positive test, but the reactions are usually only 5 to 10 mm and tend to decrease with time. People with PPD reactions of 15 mm or more are assumed to be infected with M. tuberculosis even if they have received the BCG vaccine. A positive skin test reverts to negative in about 5% to 10% of people. Reversion to negative is more common in the United States now than many years ago because now a person is less likely to be exposed to the organism and therefore less likely to receive a boost to the immune system.
The skin test itself does not induce a positive response in a person who has not been exposed to the organism. It can, however, “boost” a weak or negative response in a person who has been exposed to produce a positive reaction. The clinical implications of this “booster effect” are beyond the scope of this book.
Tuberculin reactivity is mediated by the cellular arm of the immune system; it can be transferred by CD4-positive T cells but not by serum. Infection with measles virus can suppress cell-mediated immunity, resulting in a loss of tuberculin skin test reactivity and, in some instances, reactivation of dormant organisms and clinical disease.
A gene called Nramp determines natural resistance to tuberculosis. People who have mutations in the Nramp gene have a much higher rate of clinical tuberculosis than those with a normal allele. The NRAMP protein is located in the membrane of the phagosome in macrophages and plays an important role in killing the organism within the phagosome.
Clinical Findings
Clinical findings are protean; many organs can be involved. Fever, fatigue, night sweats, and weight loss are common. Pulmonary tuberculosis causes cough and hemoptysis. Scrofula is mycobacterial cervical lymphadenitis that presents as swollen, nontender lymph nodes, usually unilaterally. M. tuberculosis causes most cases of scrofula, but nontuberculous Mycobacteria, such as Mycobacterium scrofulaceum, can also cause scrofula. Lymphadenitis is the most common extrapulmonary manifestation of tuberculosis. Patients infected with human immunodeficiency virus (HIV) are more likely to have multifocal lymphadenitis than those not infected with HIV.
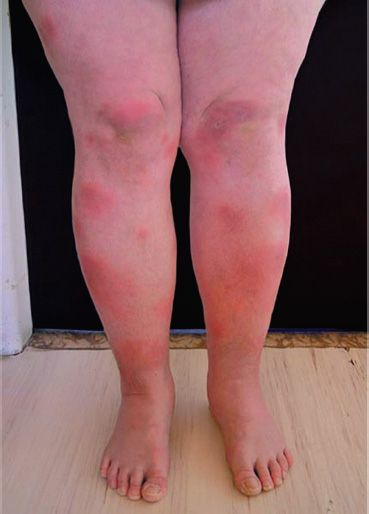
Erythema nodosum, characterized by tender nodules along the extensor surfaces of the tibia and ulna, is a manifestation of primary infection seen in patients who are controlling the infection with a potent cell-mediated response (Figure 21–3). Miliary tuberculosis is characterized by multiple disseminated lesions that resemble millet seeds. Tuberculous meningitis and tuberculous osteomyelitis, especially vertebral osteomyelitis (Pott’s disease), are important disseminated forms.
FIGURE 21–3 Erythema nodosum. Note erythematous nodules over the anterior surface of the tibia bilaterally. (Courtesy of Dr. Hanus Rozsypal.)
Gastrointestinal tuberculosis is characterized by abdominal pain and diarrhea accompanied by more generalized symptoms of fever and weight loss. Intestinal obstruction or hemorrhage may occur. The ileocecal region is the site most often involved. Tuberculosis of the gastrointestinal tract can be caused by either M. tuberculosis when it is swallowed after being coughed up from a lung lesion or by M. bovis when it is ingested in unpasteurized milk products. Oropharyngeal tuberculosis typically presents as a painless ulcer accompanied by local adenopathy.
In renal tuberculosis, dysuria, hematuria, and flank pain occur. “Sterile pyuria” is a characteristic finding. The urine contains white blood cells, but cultures for the common urinary tract bacterial pathogens show no growth. However, mycobacterial cultures are often positive.
Note that most (approximately 90%) infections with M. tuberculosis are asymptomatic. Asymptomatic infections, also known as latent infections, can reactivate and cause symptomatic tuberculosis. Although there may be some differences in the virulence between strains of the organism, the most important determinant of whether overt disease occurs is the adequacy of the host’s cell-mediated immune (CMI) response. For example, AIDS patients have a very high rate of reactivation of prior asymptomatic infection and of rapid progression of the disease. In these patients, untreated disease caused by M. tuberculosis has a 50% mortality rate. Furthermore, administration of infliximab (Remicade), a monoclonal antibody that neutralizes tumor necrosis factor (TNF), has activated latent tuberculosis in some patients. Remicade is used in the treatment of rheumatoid arthritis (see Chapter 66). Diabetics also are predisposed to reactivation and progression of disease.
In some patients with AIDS who are infected with M. tuberculosis, treating the patient with highly active antiretroviral therapy (HAART) causes an exacerbation of symptoms. This phenomenon is called immune reconstitution inflammatory syndrome (IRIS). The explanation of the exacerbation of symptoms is that HAART increases the number of CD4 cells, which increases the inflammatory response. To prevent this, patients should be treated for the underlying infection before starting HAART.
Laboratory Diagnosis
Acid-fast staining of sputum or other specimens is the usual initial test (Figure 21–1). Either the Kinyoun version of the acid-fast stain or the older Ziehl-Neelsen version can be used. For rapid screening purposes, auramine stain, which can be visualized by fluorescence microscopy, is used.
After digestion of the specimen by treatment with NaOH and concentration by centrifugation, the material is cultured on special media, such as Löwenstein-Jensen agar, for up to 8 weeks. It will not grow on a blood agar plate. In liquid BACTEC medium, radioactive metabolites are present, and growth can be detected by the production of radioactive carbon dioxide in about 2 weeks. A liquid medium is preferred for isolation because the organism grows more rapidly and reliably than it does on agar. If growth in the culture occurs, the organism can be identified by biochemical tests. For example, M. tuberculosis produces niacin, whereas almost no other mycobacteria do. It also produces catalase.
Nucleic acid amplification tests can be used to detect the presence of M. tuberculosis directly in clinical specimens such as sputum. Tests are available that detect either the ribosomal RNA or the DNA of the organism. These tests are highly specific, but their sensitivity varies. In sputum specimens that are acid-fast stain positive, the sensitivity is high, but in “smear-negative” sputums, the sensitivity is significantly lower. These tests are quite useful in deciding whether to initiate therapy prior to obtaining the culture results.
Because drug resistance, especially to isoniazid (see later), is a problem, susceptibility tests should be performed. However, the organism grows very slowly, and susceptibility tests usually take several weeks, which is too long to guide the initial choice of drugs. To address this problem, molecular tests are available, which detect mutations in the chromosomal genes that encode either the catalase gene that mediates resistance to isoniazid or the RNA polymerase gene that mediates resistance to rifampin. The luciferase assay, which can detect drug-resistant organisms in a few days, is also used. Luciferase is an enzyme isolated from fireflies that produces flashes of light in the presence of adenosine triphosphate (ATP). If the organism isolated from the patient is resistant, it will not be damaged by the drug (i.e., it will make a normal amount of ATP), and the luciferase will produce the normal amount of light. If the organism is sensitive to the drug, less ATP will be made and less light produced.
There are two approaches to the diagnosis of latent infections. One is the PPD skin test as described in the “Immunity & Hypersensitivity” section earlier in this chapter. Because there are problems both in the interpretation of the PPD test and with the person returning for the skin test to be read, a quantifiable laboratory-based test is valuable. This laboratory test is an interferon-γ release assay (IGRA), and there are two versions available: Quantiferon-TB and T-spot.TB. In this assay, blood cells from the patient are exposed to antigens from M. tuberculosis, and the amount of interferon-γ released from the cells is measured. The sensitivity and specificity of the IGRA are as good as the PPD skin test. Because the antigens used in the test are specific for M. tuberculosis and are not present in BCG, the test is not influenced by whether a person has been previously immunized with the BCG vaccine.
Treatment & Resistance
Multidrug therapy is used to prevent the emergence of drug-resistant mutants during the long (6- to 9-month) duration of treatment. (Organisms that become resistant to one drug will be inhibited by the other.) Isoniazid (INH), a bactericidal drug, is the mainstay of treatment. Treatment for most patients with pulmonary tuberculosis is with three drugs: INH, rifampin, and pyrazinamide. INH and rifampin are given for 6 months, but pyrazinamide treatment is stopped after 2 months. A somewhat different regimen can also be used. A convenient way to remember that regimen is to give four drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) for 2 months and two drugs (isoniazid and rifampin) for 4 months. In patients who are immunocompromised (e.g., AIDS patients), who have disseminated disease, or who are likely to have INH-resistant organisms, a fourth drug, ethambutol, is added, and all four drugs are given for 9 to 12 months.
Although therapy is usually given for months, the patient’s sputum becomes noninfectious within 2 to 3 weeks. The necessity for protracted therapy is attributed to (1) the intracellular location of the organism; (2) caseous material, which blocks penetration by the drug; (3) the slow growth of the organism; and (4) metabolically inactive “persisters” within the lesion. Because metabolically inactive organisms may not be killed by antitubercular drugs, treatment may not eradicate the infection, and reactivation of the disease may occur in the future.
Lymphadenitis, including cervical lymphadenitis (scrofula) caused by M. tuberculosis, should be treated with the drug regimens described earlier for disseminated disease. Scrofula caused by M. scrofulaceum can be treated by surgical excision of the single cervical lymph node, but alternative approaches exist. A complete discussion of these is beyond the scope of this book.
Treatment of latent (asymptomatic) infections consists of INH taken for 6 to 9 months or INH plus rifapentine for 3 months. This approach is most often used in asymptomatic patients whose PPD skin test recently converted to positive. The risk of symptomatic infection is greatest within the first 2 years after infection, so INH is particularly indicated for these “recent converters.” INH is also used in children exposed to patients with symptomatic tuberculosis. Patients who receive INH should be evaluated for drug-induced hepatitis, especially those over the age of 35 years. Rifampin can be used in those exposed to INH-resistant strains. A combination of rifampin and pyrazinamide should not be used because it causes a high rate of severe liver injury.
Resistance to INH and other antituberculosis drugs is being seen with increasing frequency in the United States, especially in immigrants from Southeast Asia and Latin America. Strains of M. tuberculosis resistant to multiple drugs (MDR strains) have emerged, primarily in AIDS patients. The most common pattern is resistance to both INH and rifampin, but some isolates are resistant to three or more drugs. The treatment of MDR organisms usually involves the use of four or five drugs, including ciprofloxacin, amikacin, ethionamide, and cycloserine. The precise recommendations depend on the resistance pattern of the isolate and are beyond the scope of this book.
In 2013, a new drug, bedaquiline, was approved for the treatment of MDR strains. It should be used in combination with other drugs, not as monotherapy. It is a diarylquinoline that inhibits an ATP synthase unique to M. tuberculosis.
Previous treatment for tuberculosis predisposes to the selection of these MDR organisms. Noncompliance (i.e., the failure of patients to complete the full course of therapy) is a major factor in allowing the resistant organisms to survive. One approach to the problem of noncompliance is directly observed therapy (DOT), in which health care workers observe the patient taking the medication.
The strains of M. tuberculosis resistant to INH, rifampin, a fluoroquinolone, and at least one additional drug are called extensively drug-resistant (XDR) strains. XDR strains emerged in 2005 among HIV-infected patients in South Africa.
Prevention
The incidence of tuberculosis began to decrease markedly even before the advent of drug therapy in the 1940s. This is attributed to better housing and nutrition, which have improved host resistance. At present, prevention of the spread of the organism depends largely on the prompt identification and adequate treatment of patients who are coughing up the organism. The use of masks and other respiratory isolation procedures to prevent spread to medical personnel is also important. Contact tracing of individuals exposed to patients with active pulmonary disease who are coughing should be done.
An important component of prevention is the use of the PPD skin test to detect recent converters and to institute treatment for latent infections as described earlier. Groups that should be screened with the PPD skin test include people with HIV infection, close contacts of patients with active tuberculosis, low-income populations, alcoholics and intravenous drug users, prison inmates, and foreign-born individuals from countries with a high incidence of tuberculosis.
Because there are some problems associated with PPD skin tests, such as the measurement and the interpretation of results and the inconvenience of the patient having to return for the skin test to be read, a laboratory test to detect latent infections was developed. This test, called Quantiferon-TB (QFT), measures the amount of interferon-γ released from the patient’s lymphocytes after exposure to PPD in cell culture. QFT requires only a single blood specimen and determines the amount of interferon-γ by an enzyme-linked immunosorbent assay (ELISA) test.
BCG vaccine can be used to induce partial resistance to tuberculosis. The vaccine contains a strain of live, attenuated M. bovis called bacillus Calmette-Guérin. The vaccine is effective in preventing the appearance of tuberculosis as a clinical disease, especially in children, although it does not prevent infection by M. tuberculosis. However, a major problem with the vaccine is its variable effectiveness, which can range from 0% to 70%. It is used primarily in areas of the world where the incidence of the disease is high. It is not usually used in the United States because of its variable effectiveness and because the incidence of the disease is low enough that it is not cost-effective.
The skin test reactivity induced by the vaccine given to children wanes with time, and the interpretation of the skin test reaction in adults is not altered by the vaccine. For example, skin test reactions of 10 mm or more should not be attributed to the vaccine unless it was administered recently. In the United States, use of the vaccine is limited to young children who are in close contact with individuals with active tuberculosis and to military personnel. BCG vaccine should not be given to immunocompromised people because the live BCG organisms can cause disseminated disease.
BCG vaccine is also used to treat bladder cancer. The vaccine is instilled into the bladder and serves to nonspecifically stimulate cell-mediated immunity, which can inhibit the growth of the carcinoma cells.
Pasteurization of milk and destruction of infected cattle are important in preventing intestinal tuberculosis.
ATYPICAL MYCOBACTERIA
Several species of mycobacteria are characterized as atypical, because they differ in certain respects from the prototype, M. tuberculosis. For example, atypical mycobacteria are widespread in the environment and are not pathogenic for guinea pigs, whereas M. tuberculosis is found only in humans and is highly pathogenic for guinea pigs. The atypical mycobacteria are sometimes called mycobacteria other than tuberculosis (MOTTs).
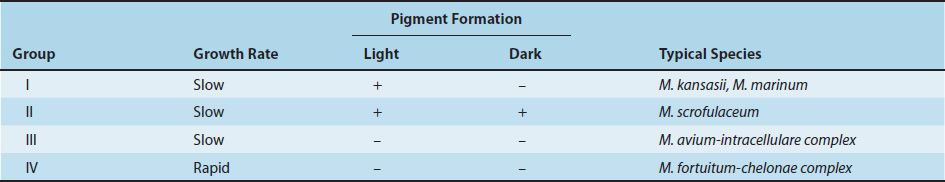
The atypical mycobacteria are classified into four groups according to their rate of growth and whether they produce pigment under certain conditions (Table 21–3). The atypical mycobacteria in groups I, II, and III grow slowly, at a rate similar to that of M. tuberculosis, whereas those in group IV are “rapid growers,” producing colonies in fewer than 7 days. Group I organisms produce a yellow-orange–pigmented colony only when exposed to light (photochromogens), whereas group II organisms produce the pigment chiefly in the dark (scotochromogens). Group III mycobacteria produce little or no yellow-orange pigment, irrespective of the presence or absence of light (nonchromogens).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree