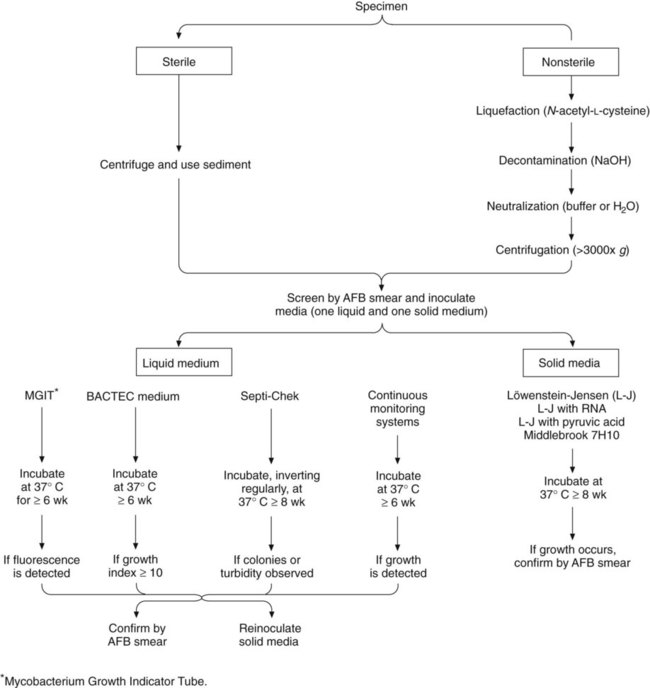
1. Describe the general characteristics of the Mycobacterium spp., including oxygen requirements, staining patterns and cell morphology, artificial media required for cultivation and growth, and pigmentation. 2. Explain the chemical composition of the bacterial cell wall. 3. Explain the microscopic staining characteristics of Mycobacterium spp. using the Gram stain and acid-fast staining methods. 4. List the most common pathogenic species in the Mycobacterium genus and state the natural habitat, mode of transmission, and reservoir for each. 5. Differentiate M. tuberculosis clinical infections based on the signs and symptoms of the following: primary infection, latent infection, disseminated infection, and reactivation. 6. Compare the current safety and containment methods recommended for handling mycobacterial infectious materials and routine bacteriology in a diagnostic laboratory. 7. Describe the purified protein derivative (PPD; also referred to as the tuberculin skin test). What is the significance of a positive result? 8. List the clinical specimens acceptable for recovery of mycobacteria and describe the limitations of recovery from each type of specimen. 9. Justify the use of DNA probes and molecular sequencing or amplification methods to identify Mycobacterium spp. 10. Evaluate the effectiveness of the staining procedures—Kinyoun, Ziehl-Neelsen, and fluorescent staining (auramine-rhodamine or acridine orange)—for identifying mycobacteria. 11. Describe the requirements for using digestion and decontamination procedures to improve the recovery of Mycobacterium spp. 12. Explain the limitations of digestion and decontamination procedures. 13. Explain the methods commonly used for biochemical identification of Mycobacterium spp. (i.e., niacin, nitrate, urease, modified catalase, Tween 80, tellurite, arylsulfatase, thiophene-2-carboxylic acid hydrazide [TCH], and 5% NaCl tests), including the purpose, principle, and control organisms used for each. 14. Describe the role of the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) in the dissemination and/or pathogenesis of infections with Mycobacterium spp. 15. Explain the recommended susceptibility testing methods and state when susceptibility testing is required or recommended for Mycobacterium spp. For the most part, mycobacteria can be divided into two major groups, based on fundamental differences in epidemiology and association with disease: those belonging to the Mycobacterium tuberculosis complex and those referred to as nontuberculous mycobacteria (NTM) (Box 43-1). M. tuberculosis is the cause of most cases of human tuberculosis, particularly in developed countries. An estimated 1.7 billion people, or one third of the world’s population, are infected with M. tuberculosis. This reservoir of infected individuals results in 8 million new cases of tuberculosis and 2.9 million deaths annually. Tuberculosis continues to be a public health problem in the United States. An additional complicating factor in the management of tuberculosis is the increasing incidence of co-infection with the human immunodeficiency virus (HIV). HIV-associated tuberculosis remains a significant challenge to world health, with an estimated 1.1 million individuals living with HIV-associated tuberculosis. In the United States, tuberculosis typically is found among the poor, homeless, intravenous (IV) drug users, alcoholics, the elderly, or medically underserved populations. Although the organisms belonging to the M. tuberculosis complex have numerous characteristics in common, including extreme genetic homogeneity, they differ in certain epidemiologic aspects (Table 43-1). TABLE 43-1 Epidemiology of Organisms Belonging to M. tuberculosis Complex That Cause Human Infections *Infection occasionally can occur through the gastrointestinal tract or skin. †The incidence has decreased significantly in developed countries since the introduction of universal pasteurization of milk and milk products and the institution of effective control programs for cattle. ‡Can be transmitted human to human, animal to human, and human to animal. The pathogenesis of tuberculosis caused by organisms of the M. tuberculosis complex is discussed in Chapter 69. Inhalation of a single viable organism has been shown to lead to infection, although close contact is usually necessary. Of those who become infected with M. tuberculosis, 15% to 20% develop disease. The disease usually occurs some years after the initial infection, when the patient’s immune system breaks down for some reason other than the presence of tuberculosis bacilli in the lung. In a small percentage of infected hosts, the disease becomes systemic, affecting a variety of organs. The NTM include all mycobacterial species that do not belong to M. tuberculosis complex. Currently, approximately 130 species of nontuberculous mycobacteria have been recognized. The members of this large group of mycobacteria have been known by several names (Box 43-2). Significant geographic variability is seen both in the prevalence of and the species responsible for NTM disease. As previously mentioned, NTM are present everywhere in the environment and sometimes colonize the skin and respiratory and gastrointestinal tracts of healthy individuals. Little is known about how infection is acquired, but some mechanisms appear to be trauma, inhalation of infectious aerosols, and ingestion; a few diseases are nosocomial or are acquired as an iatrogenic infection. In contrast to M. tuberculosis complex, NTM are not usually transmitted from person to person, nor does isolation of these organisms necessarily mean they are associated with a disease process. Interpretation of a positive NTM culture is complicated, because these organisms are widely distributed in nature, their pathogenic potential varies greatly from one species to another, and humans can be colonized by these mycobacteria without necessarily developing infection or disease. With few exceptions, little is known about the pathogenesis of infections caused by these bacterial agents. In 1959 Runyon1 classified NTM into four groups (Runyon groups I to IV) based on the phenotypic characteristics of the various species, most notably the growth rate and colonial pigmentation (Table 43-2). Runyon’s system first categorizes the slow-growing NTM (Runyon groups I to III) and then the rapid-growers (Runyon group IV). One other NTM, M. leprae, which cannot be cultivated on artificial media, is also reviewed. (As with many classification schemes, the Runyon classification does not always hold true. For example, some NTM can be either a photochromogen or a nonphotochromogen.) TABLE 43-2 Runyon Classification of Nontuberculous Mycobacteria (NTM) The photochromogens (Table 43-3) are slow-growing NTM that produce colonies that require light to form pigment. TABLE 43-3 Characteristics of Nontuberculous Mycobacteria—Photochromogens The scotochromogens (Table 43-4) are slow-growing NTM that produce pigmented colonies whether grown in the dark or the light. The epidemiology of the potentially pathogenic scotochromogens has not been definitively described. In contrast to potentially pathogenic nonphotochromogens, these agents are rarely recovered in the clinical laboratory. TABLE 43-4 Characteristics of Nontuberculous Mycobacteria—Scotochromogens NA, Not applicable. The nonphotochromogens (Table 43-5) are slow-growing NTM that produce unpigmented colonies whether grown in the dark or the light. Of the organisms in this group, M. terrae complex (M. terrae, M. triviale, and M. nonchromogenicum) and M. gastri are considered nonpathogenic for humans. The other nonphotochromogens are considered potentially pathogenic, and many are frequently recovered in the clinical laboratory. The nonphotochromogens belonging to Mycobacterium avium complex are frequently isolated in the clinical laboratory and are able to cause infection in the human host. TABLE 43-5 AIDS, Acquired immunodeficiency syndrome; HIV, human immunodeficiency virus. *Disseminated disease can involve multiple sites, such as bone marrow, lungs, liver, lymph nodes. Several other mycobacterial species that are considered nonphotochromogens are potentially pathogenic in humans. The epidemiology and spectrum of disease for these organisms are summarized in Table 43-5. In addition to the species in this table, other, newer species of mycobacteria that are nonphotochromogens have been described, such as M. celatum and M. conspicuum. These newer agents appear to be potentially pathogenic in humans. The large group of organisms that constitute the RGM is divided into six major groups of potentially pathogenic species, based on pigmentation and molecular studies (see Box 43-1). Unlike the majority of other mycobacteria, most rapid-growers can grow on routine bacteriologic media and on media specific for cultivation of mycobacteria. On Gram staining, these organisms appear as weakly gram-positive rods resembling diphtheroids. The spectrum of disease caused by the most commonly encountered rapid-growers is summarized in Table 43-6. The most common infection associated with RGM is posttraumatic wound infection. An increase in wound infections has been associated with planktonic M. abscessus, which can be identified as a rough colonial phenotype on artificial media; these organisms are capable of infecting macrophages. The smooth colonial phenotype typically is identified in biofilms and lacks infectivity. TABLE 43-6 Common Types of Infections Caused by Rapidly Growing Mycobacteria Early morning voided urine specimens (40 mL minimum) in sterile containers should be submitted daily for at least 3 days. The collection procedure is the same as for collecting a clean-catch midstream urine specimen (see Chapter 73). The 24-hour urine specimen is undesirable because of excessive dilution, higher contamination, and difficulty in concentrating. Catheterization should be used only if a midstream voided specimen cannot be collected. Immunocompromised patients, particularly those infected with HIV, can have disseminated mycobacterial infection; most of these infections are caused by M. avium complex. A blood culture positive for MAC is always associated with clinical evidence of disease. Recovery of mycobacteria is improved with blood collection in either a broth or the Isolator lysis-centrifugation system (see Chapter 68). Some studies have indicated that the lysis-centrifugation system is advantageous, because quantitative data can be obtained with each blood culture; in patients with AIDS, quantitation of such organisms can be used to monitor therapy and determine the prognosis. However, the necessity of quantitative blood cultures remains unclear. Processing to recover acid-fast bacilli from clinical specimens involves several complex steps, each of which must be carried out with precision. Specimens from sterile sites can be inoculated directly to media (small volume) or concentrated to reduce volume. Other specimens require decontamination and concentration. A processing scheme is shown in Figure 43-1, and the procedures are explored in detail in the following discussions.
Mycobacteria
Mycobacterium Tuberculosis Complex
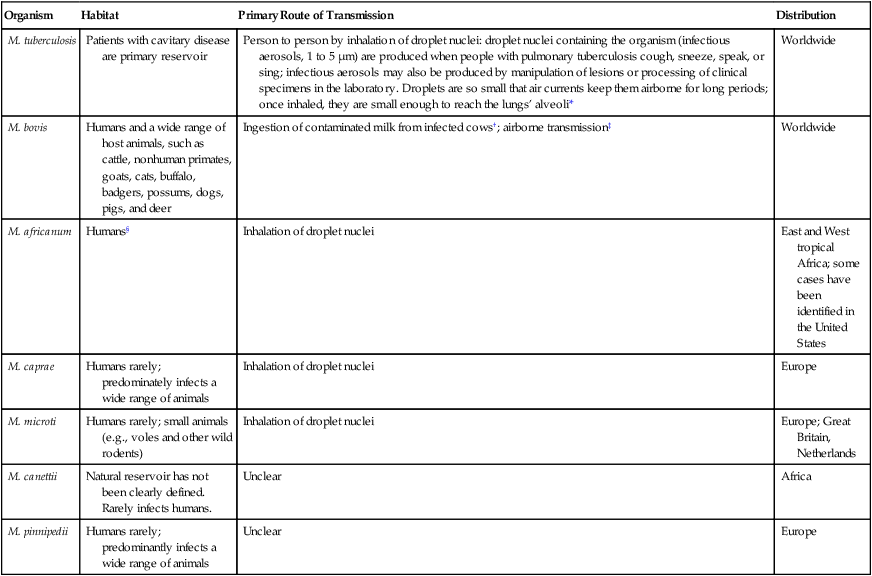
Epidemiology and Pathogenesis
Epidemiology
Organism
Habitat
Primary Route of Transmission
Distribution
M. tuberculosis
Patients with cavitary disease are primary reservoir
Person to person by inhalation of droplet nuclei: droplet nuclei containing the organism (infectious aerosols, 1 to 5 µm) are produced when people with pulmonary tuberculosis cough, sneeze, speak, or sing; infectious aerosols may also be produced by manipulation of lesions or processing of clinical specimens in the laboratory. Droplets are so small that air currents keep them airborne for long periods; once inhaled, they are small enough to reach the lungs’ alveoli*
Worldwide
M. bovis
Humans and a wide range of host animals, such as cattle, nonhuman primates, goats, cats, buffalo, badgers, possums, dogs, pigs, and deer
Ingestion of contaminated milk from infected cows†; airborne transmission‡
Worldwide
M. africanum
Humans§
Inhalation of droplet nuclei
East and West tropical Africa; some cases have been identified in the United States
M. caprae
Humans rarely; predominately infects a wide range of animals
Inhalation of droplet nuclei
Europe
M. microti
Humans rarely; small animals (e.g., voles and other wild rodents)
Inhalation of droplet nuclei
Europe; Great Britain, Netherlands
M. canettii
Natural reservoir has not been clearly defined. Rarely infects humans.
Unclear
Africa
M. pinnipedii
Humans rarely; predominantly infects a wide range of animals
Unclear
Europe
Pathogenesis
Nontuberculous Mycobacteria
Runyon Group Number
Group Name
Description
I
Photochromogens
NTM colonies that develop pigment on exposure to light after being grown in the dark and take longer than 7 days to appear on solid media
II
Scotochromogens
NTM colonies that develop pigment in the dark or light and take longer than 7 days to appear on solid media
III
Nonphotochromogens
NTM colonies that are nonpigmented regardless of whether they are grown in the dark or light and take longer than 7 days to appear on solid media
IV
Rapid growers
NTM colonies that grow on solid media and take fewer than 7 days to appear
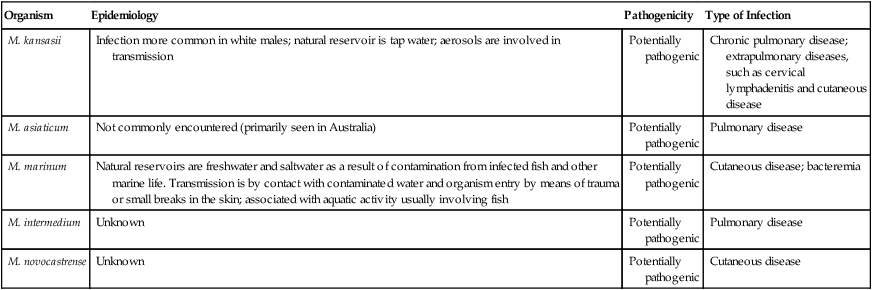
Slow-Growing Nontuberculous Mycobacteria
Photochromogens
Organism
Epidemiology
Pathogenicity
Type of Infection
M. kansasii
Infection more common in white males; natural reservoir is tap water; aerosols are involved in transmission
Potentially pathogenic
Chronic pulmonary disease; extrapulmonary diseases, such as cervical lymphadenitis and cutaneous disease
M. asiaticum
Not commonly encountered (primarily seen in Australia)
Potentially pathogenic
Pulmonary disease
M. marinum
Natural reservoirs are freshwater and saltwater as a result of contamination from infected fish and other marine life. Transmission is by contact with contaminated water and organism entry by means of trauma or small breaks in the skin; associated with aquatic activity usually involving fish
Potentially pathogenic
Cutaneous disease; bacteremia
M. intermedium
Unknown
Potentially pathogenic
Pulmonary disease
M. novocastrense
Unknown
Potentially pathogenic
Cutaneous disease
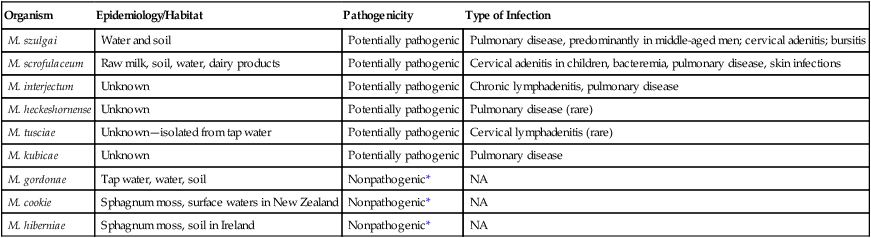
Scotochromogens
Organism
Epidemiology/Habitat
Pathogenicity
Type of Infection
M. szulgai
Water and soil
Potentially pathogenic
Pulmonary disease, predominantly in middle-aged men; cervical adenitis; bursitis
M. scrofulaceum
Raw milk, soil, water, dairy products
Potentially pathogenic
Cervical adenitis in children, bacteremia, pulmonary disease, skin infections
M. interjectum
Unknown
Potentially pathogenic
Chronic lymphadenitis, pulmonary disease
M. heckeshornense
Unknown
Potentially pathogenic
Pulmonary disease (rare)
M. tusciae
Unknown—isolated from tap water
Potentially pathogenic
Cervical lymphadenitis (rare)
M. kubicae
Unknown
Potentially pathogenic
Pulmonary disease
M. gordonae
Tap water, water, soil
Nonpathogenic*
NA
M. cookie
Sphagnum moss, surface waters in New Zealand
Nonpathogenic*
NA
M. hiberniae
Sphagnum moss, soil in Ireland
Nonpathogenic*
NA
Nonphotochromogens
Organism
Epidemiology
Type of Infection
M. avium complex
Environmental sources, including natural waters, and soil
Patients without AIDS: Pulmonary infections in patients with preexisting pulmonary disease; cervical lymphadenitis; and disseminated disease* in immunocompromised patients who are HIV negative
Patients with AIDS: Disseminated disease
M. xenopi†
Water, especially hot water taps in hospitals; believed to be transmitted in aerosols
Primarily pulmonary infections in adults; less common, extrapulmonary infections (bone, lymph nodes, sinus tract) and disseminated disease
M. ulcerans
Stagnant tropical waters; also harbored in an aquatic insect’s salivary glands; infections occur in tropical or temperate climates
Indolent cutaneous and subcutaneous infections (African Buruli ulcer or Australian Bairnsdale ulcer)
M. malmoense
Most cases from England, Wales, and Sweden. Rarely isolated from patients infected with HIV. Little is known about epidemiology; to date, isolated only from humans and captured armadillos
Chronic pulmonary infections, primarily in patients with preexisting disease; cervical lymphadenitis in children; less common, infections of the skin or bursae
M. genovense
Isolated from pet birds and dogs. Mode of acquisition unknown
Disseminated disease in patients with AIDS (wasting disease characterized by fever, weight loss, hepatosplenomegaly, anemia)
M. haemophilum
Unknown
Disseminated disease; cutaneous infections in immunosuppressed adults; mild and limited skin infections in preadolescence or early adolescence; cervical lymphadenitis in children
M. heidelbergense
Unknown
Lymphadenitis in children; also isolated from sputum, urine, and gastric aspirate
M. shimoidei
To date has not been isolated from environmental sources; few case reports, but widespread geographically
Tuberculosis-like pulmonary infection; disseminated disease
M. simiae
Tap water and hospital water tanks; rarely isolated
Tuberculosis-like pulmonary infection
Other Nonphotochromogens.
Rapidly Growing Nontuberculous Mycobacteria (RGM)
General Characteristics
Spectrum of Disease
Organism
Common Types of Infection
M. abscessus subsp. abscessus
Disseminated disease, primarily in immunocompromised individuals; skin and soft tissue infections; pulmonary infections; postoperative infections
M. fortuitum
Postoperative infections in breast augmentation and median sternotomy; skin and soft tissue infections; pulmonary infections, usually single. localized lesions.
Central nervous system (CNS) disease is rare but has high morbidity and mortality
M. chelonae
Skin and soft tissue infections, postoperative wound infections, keratitis
Less Common Types of Infection (More Than 10 Cases)
M. peregrinum
Skin and soft tissue infections; bacteremia
M. mucogenicum
Posttraumatic wound infections, catheter-related sepsis, health care associated
M. smegmatis
Skin or soft tissue infections; less frequently, pulmonary infections
M. abscessus subsp. bolletii
Health care–associated infections, skin and soft tissue infections, pulmonary infections
M. boenickei
Bone and joint infections
M. canariasense
Bacteremia
M. cosmeticum
Pulmonary and urosepsis
M. goodii
Bone and joint infections, osteomyelitis
M. houstonense
Bone and joint infections
M. immunogenum
Hypersensitivity pneumonitis
M. neoaurum (closely related to M. lacticola)
Catheter-related sepsis
M. porcinum
Surgical site infection
M. senegalense
Catheter-related sepsis
Rare Infections (Fewer Than 10 Cases)
M. aubagnense
Various opportunistic health care–associated infections
M. brisbanense
Various opportunistic health care–associated infections
M. brumae
Various opportunistic health care–associated infections
M. elephantis
Various opportunistic health care–associated infections
M. mageritense
Skin and soft tissue infections
M. monacense
Various opportunistic health care–associated infections
M. moriokaense
Various opportunistic health care–associated infections
M. neworleansense
Various opportunistic health care–associated infections
M. novocastrense
Various types of opportunistic health care–associated infections
M. phocaicum
Catheter-related sepsis
M. septicum
Various opportunistic health care–associated infections
M. setense
Bone and joint infections
M. wolinskyi
Skin and soft tissue infections, bone infection, osteomyelitis
Laboratory Diagnosis of Mycobacterial Infections
Specimen Collection and Transport
Urine Specimens
Blood Specimens
Specimen Processing
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mycobacteria