INTRODUCTION
LEARNING OBJECTIVES
After studying this chapter you should understand:
The causes and consequences of bone disease, renal dysfunction, and immunodeficiency in multiple myeloma.
The diagnosis and treatment of multiple myeloma.
The major pathologic and clinical features of the other plasma cell and lymphoplasmacytic neoplasms.
Neoplasms composed of plasma cells have special biological and clinical features related to their capacity to secrete complete or partial immunoglobulin (Ig) proteins. By far the most important of these tumors is multiple myeloma, which is diagnosed in about 15,000 patients per year in the United States. It is a tumor of older adults, with a median age at diagnosis of 69 years. For unknown reasons, it is more common in people of African descent.
MULTIPLE MYELOMA
As the name implies, at diagnosis multiple myeloma typically involves multiple bones of the axial skeleton, particularly the vertebrae, skull, proximal long bones of the extremities, and the ribs. The pathophysiology of the disease is primarily related to four factors:
Pathogenic antibodies or antibody fragments. Normal antibodies are composed of two heavy chains encoded by the IgH locus and two light chains, which may be encoded by either the Ig kappa or the Ig lambda locus. Multiple myeloma cells most commonly secrete IgG or IgA antibodies. However, in addition to complete antibodies, neoplastic plasma cells usually also secrete free, unpaired Ig light chains; indeed, in about 20% of cases, only light chains are secreted. The small size of the light chains (around 25 kDa) permits them to pass from the blood through the filtration slits of the renal glomeruli and into the renal tubules. Once in the urinary space, Ig light chains are toxic to renal epithelial cells and tend to form precipitates and obstructive casts, both of which contribute to renal dysfunction (Fig. 24-1). Free light chains, particularly lambda light chains, are also prone to form amyloid, fibrillar deposits that may be found in the renal glomeruli (Fig. 24-2) and the perivascular spaces of many tissues, including the liver, spleen, and heart. Renal amyloidosis often causes nephrotic syndrome, the spilling of albumin and other plasma proteins into the urine. Alternatively, instead of forming amyloid, free light chains sometimes accumulate in amorphous linear deposits in the kidney and other tissues that produce light chain deposition disease (Fig. 24-3). In more than 85% of cases these light chain deposits are composed of kappa light chains. Light chain deposition disease most commonly presents as renal dysfunction but can also cause clinically significant hepatic or cardiac failure.
Bone resorption. Bone resorption is caused by tumor-derived factors such as MIP1α and modulators of the Wnt signaling pathway, which act together to suppress osteoblast function and enhance osteoclast function (Fig. 24-4). Tilting this balance leads to marked thinning of bone, setting the stage for pathologic fractures, bone pain, and often hypercalcemia.
Suppression of humoral immunity. The proliferation of malignant plasma cells somehow inhibits normal B-cell function and the production of normal antibodies, resulting in an increased susceptibility to bacterial infections.
Renal failure. The deleterious effects of free light chains and light chain deposits on renal function already have been mentioned. These effects are compounded by bacterial infections (pyelonephritis) and hypercalcemia. The kidney is very vulnerable to this panoply of deleterious insults, which lead to overt renal failure in roughly 50% of myeloma patients.
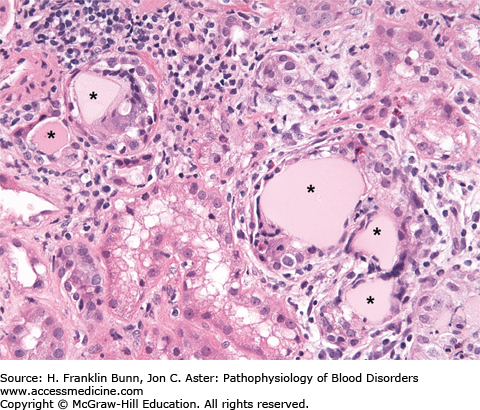
FIGURE 24-1
Myeloma kidney. The asterisks mark renal tubules that are obstructed by light pink casts composed of immunoglobulin light chains mixed with a number of other proteins. The casts have elicited an inflammatory response consisting of macrophages, lymphocytes, and a few eosinophils. (Courtesy of Dr. Helmut Rennke, Department of Pathology, Brigham and Women’s Hospital.)
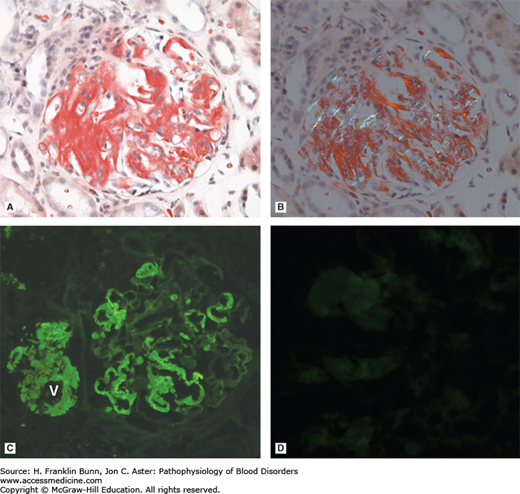
FIGURE 24-2
Light chain amyloidosis. A) The capillary walls of this glomerulus contain amorphous deposits that stain positive with the dye Congo red, which selectively binds to amyloid. B) When viewed under polarized light, amyloid stained with Congo red appears green (so-called apple green birefringence), a highly characteristic feature. C, D) Immunofluorescent staining carried out with antibodies specific for lambda (C) and kappa (D) immunoglobulin light chains demonstrates that the amyloid is derived from lambda light chain. Note in C that amyloid is deposited both in the capillaries of the glomerulus and in the adjacent wall of the afferent vessel (V), highlighting the tendency of amyloid to deposit in vessel walls throughout the body. (Courtesy of Dr. Helmut Rennke, Department of Pathology, Brigham and Women’s Hospital.)
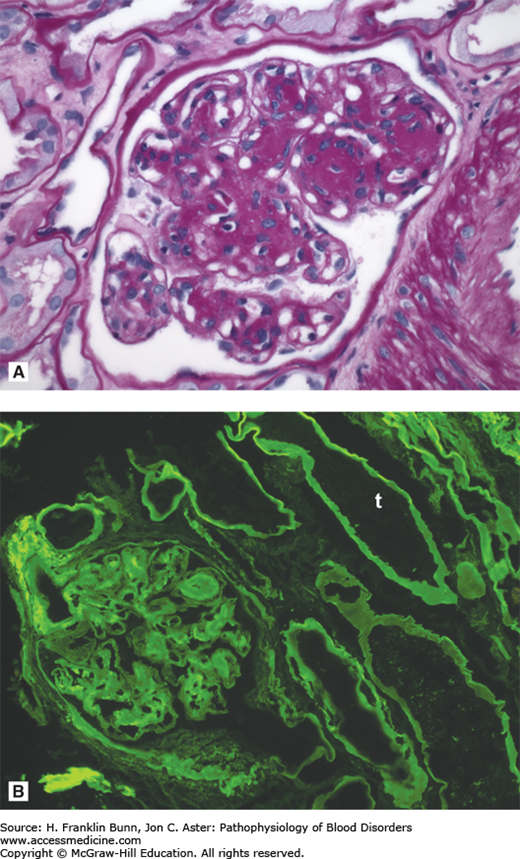
FIGURE 24-3
Light chain deposition disease. A) The glomerulus contains deposits that are positive with the periodic acid-Schiff (PAS) stain. B) Immunofluorescent staining carried out with antibodies specific for kappa and lambda immunoglobulin light chains demonstrates linear deposits of kappa light chain in the glomerulus and in the basement membranes of the renal tubules (t). (Courtesy of Dr. Helmut Rennke, Department of Pathology, Brigham and Women’s Hospital.)