Mucinous Carcinoma
When the diagnosis is restricted to tumors consisting of pure or nearly pure mucinous carcinoma, not more than 2% of mammary carcinomas fall into this category (1,2,3). Focal mucinous differentiation occurs in up to 2% of other carcinomas, which are termed infiltrating duct carcinoma with mucinous differentiation or mixed mucinous carcinoma (3,4). Mucinous carcinoma generally occurs throughout the age range of breast carcinoma. Some studies found the mean age of women with mucinous carcinoma to be older than that of patients with nonmucinous carcinoma (2,3,5). Mucinous carcinoma constitutes about 7% of carcinomas in women ages 75 or older and only 1% among those younger than age 35 (6). Immunohistochemical studies have demonstrated nuclear estrogen receptor activity in nearly 90% of cases (7).
Mucinous carcinoma can arise in ectopic breast tissue at sites that might be subject to needle biopsy, such as the axilla or vulva (8). The differential diagnosis in these unusual locations will involve metastatic carcinoma from an extrinsic primary or mucinous carcinoma arising from sweat glands. It is necessary to document the presence of benign mammary glands to consider a diagnosis of mucinous carcinoma originating in ectopic breast tissue. The presence of in situ carcinoma in this tissue will firmly establish the diagnosis of primary mucinous carcinoma.
The initial symptom of mammary mucinous carcinoma is a breast mass in the majority of patients. A pure mucinous carcinoma typically has a mammographically and sonographically circumscribed border, whereas a mixed mucinous carcinoma tends to have an irregular, indistinct margin due to fibrosis and an infiltrative growth pattern (9,10). A spiculated contour is associated with a lesser mucinous component and a higher frequency of lymph node metastases. Mammographically detected calcifications occur in a minority of the tumors (10,11), involving the invasive portion of approximately 20% of mucinous carcinomas (12,13). Calcifications may be localized in associated intraductal carcinoma.
Mucinous carcinomas have been described ranging from less than 1 cm to more than 20 cm in diameter. In 1987, a nationwide study of Danish breast cancer patients found that only 16% of mucinous carcinomas were larger than 5 cm (1). A report from Japan found that 53.6% of mucinous carcinomas were 2.0 cm or less (T1) and 37.8% were 2.1 to 5.0 cm (T2) (4).
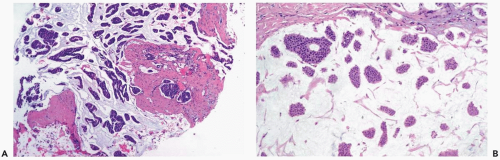
Pure mucinous carcinoma features the accumulation of extracellular mucin around invasive tumor cells (Fig. 15.1). The proportions of mucin and neoplastic epithelium vary from case to case, but the distribution within a given tumor is fairly constant. Multiple sections can be necessary to detect carcinoma cells if a tumor is composed almost entirely of extracellular mucin. The proportion of the lesion that consists of extracellular mucin in tumors classified as pure mucinous carcinomas varies from slightly less than 40% to 99.8%, with a mean percentage of 83.5 ± 14.3 (4).
Intraductal carcinoma is associated with about 75% of the lesions, generally at the periphery. It can have any of the conventional patterns (cribriform, papillary, micropapillary, comedo) (Fig. 15.2). Occasionally, mucin is evident in the cytoplasm of intraductal carcinoma cells or in the duct lumen. A difficult diagnostic problem arises in patients who have only this type of intraductal carcinoma when extravasated mucin is present in the adjacent stroma. Extravasation of mucin from intraductal carcinoma can be caused by a prior procedure or it may be spontaneous. Regardless of the mechanism by which it occurs, extravasated mucin is devoid of carcinoma cells. It may be necessary to obtain multiple recuts and to do a cytokeratin stain to determine whether epithelial cells are present in the mucin. If carcinoma cells are found in the mucin, a diagnosis of invasive mucinous carcinoma is appropriate unless there is compelling evidence to consider the alternative possibility of epithelial displacement caused by a prior procedure.
Several patterns of epithelial distribution can be found in mucinous carcinomas (14). Lesions with epithelium arranged in clusters, trabeculae, or festoons have been associated with a younger age at diagnosis than tumors in which the carcinoma cells form larger clumps (Figs. 15.1, 15.3). The latter lesions tend to have more abundant intracytoplasmic mucin, granular cytoplasm, and some of these tumor cells are argyrophilic. A micropapillary form of pure mucinous carcinoma has recently been described (15). Areas with a cribriform structure are present in many mucinous carcinomas, and a subset of the lesions
has mixed growth patterns consisting of ribbons and clumps of cells. The margin of mucinous carcinoma is determined by the extent of the mucinous component, which can be devoid of epithelial cells in the peripheral zone. The border is characterized as pushing in more than 70% of cases (13). It is difficult to recognize lymphatic tumor emboli in mucinous carcinomas because carcinoma cells suspended in mucin resemble intralymphatic carcinoma (Fig. 15.4).
has mixed growth patterns consisting of ribbons and clumps of cells. The margin of mucinous carcinoma is determined by the extent of the mucinous component, which can be devoid of epithelial cells in the peripheral zone. The border is characterized as pushing in more than 70% of cases (13). It is difficult to recognize lymphatic tumor emboli in mucinous carcinomas because carcinoma cells suspended in mucin resemble intralymphatic carcinoma (Fig. 15.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree