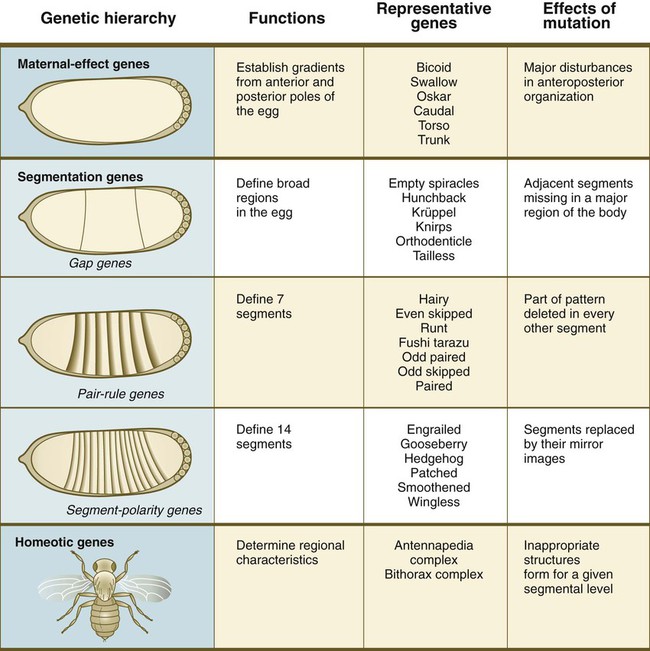
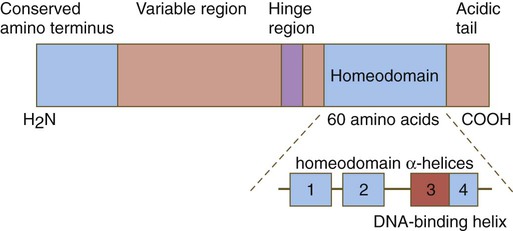
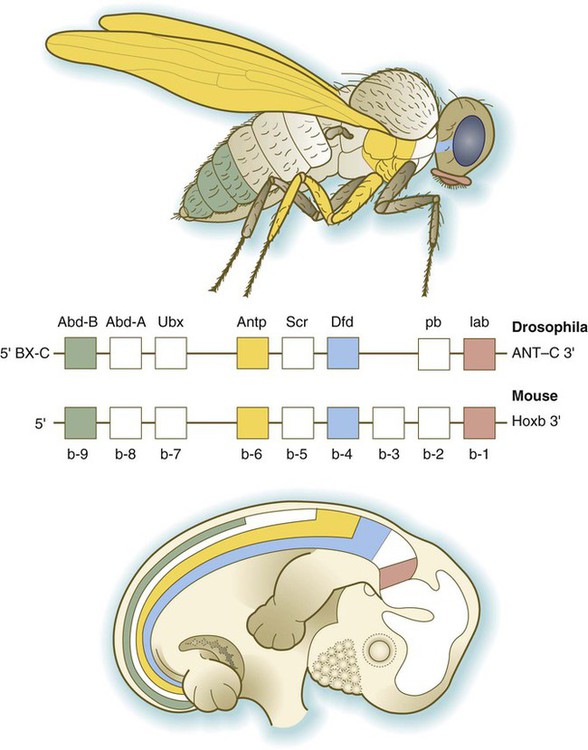
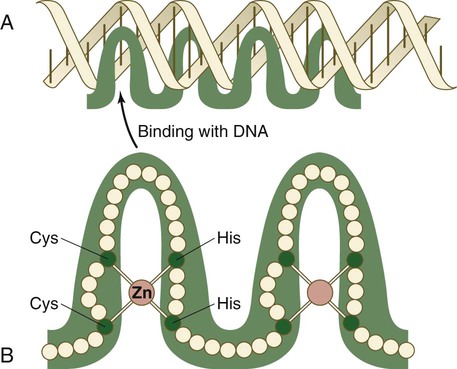
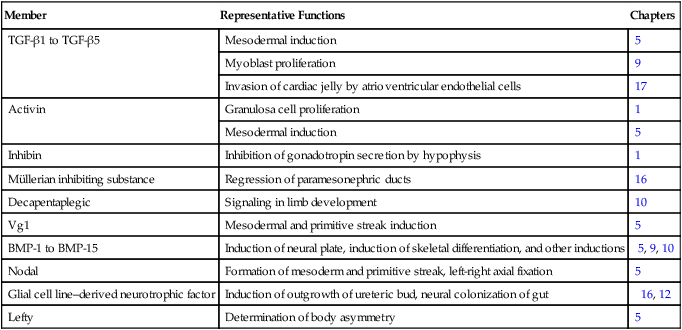
Chapter 4 One of the most important realizations has been the conservatism of the genes that guide development. Sequencing studies have shown remarkably few changes in the nucleotide bases of many developmentally regulated genes that are represented in species ranging from worms to Drosophila to humans. Because of this phylogenetic conservatism, it has been possible to identify mammalian counterparts of genes that are known from genetic studies to have important developmental functions in other species (Box 4.1).* It is also clear that the same gene may function at different periods of development and in different organs. Such reuse greatly reduces the total number of molecules that are needed to control development. Before and after birth, specific genes may be expressed in normal and abnormal processes. One of the principal themes in contemporary cancer research is the role of mutant forms of developmentally important genes (e.g., proto-oncogenes) in converting normal cells to tumor cells. From a functional standpoint, many of the important molecules that guide embryonic development can be grouped into relatively few categories. Some of them remain in the cells that produced them and act as transcription factors (Fig. 4.2). Transcription factors are proteins possessing domains that bind to the DNA of promoter or enhancer regions of specific genes. They also possess a domain that interacts with RNA polymerase II or other transcription factors and consequently regulates the amount of messenger RNA (mRNA) produced by the gene. One of the most important types of transcription factors is represented by the homeodomain proteins. These proteins contain a highly conserved homeodomain of 60 amino acids; a homeodomain is a type of helix-loop-helix region (Fig. 4.3). The 180 nucleotides in the gene that encode the homeodomain are collectively called a homeobox. Homeobox regions were first discovered in the homeotic genes of the antennapedia and bithorax complex in Drosophila (see Fig. 4.1), hence their name. This designation sometimes confuses students because, since their initial description, homeoboxes have been found in several more distantly related genes outside the homeotic gene cluster. Many other gene families contain not only a homeobox but also other conserved sequences (Fig. 4.4). The Drosophila antennapedia-bithorax complex consists of 8 homeobox-containing genes located in 2 clusters on one chromosome. Mice and humans possess at least 39 homologous homeobox genes (called Hox genes in vertebrates [HOX in humans]), which are found in 4 clusters on 4 different chromosomes (Fig. 4.5). The Hox genes on the 4 mammalian chromosomes are arranged in 13 paralogous groups. Vertebrate Hox genes play a prominent role in the craniocaudal segmentation of the body, and their spatiotemporal expression proceeds according to some remarkably regular rules. The genes are activated and expressed according to a strict sequence in the 3′ to 5′ direction, corresponding to their positions on the chromosomes. Consequently, in Drosophila and mammals, 3′ genes are expressed earlier and more anteriorly than are 5′ genes (Fig. 4.6). Mutations of Hox genes result in morphological transformations of the segmental structures in which a specific gene is normally expressed. Generally, loss-of-function mutations result in posterior-to-anterior transformations (e.g., cells of a given segment form the structural equivalent of the next most anterior segment), and gain-of-function mutations result in anterior-to-posterior structural transformations. Figure 4.7 illustrates an experiment in which injection of an antibody to a homeodomain protein into an early frog embryo resulted in the transformation of the anterior spinal cord into an expanded hindbrain. Although Hox genes were originally described to operate along the main body axis, sequential arrays of expression are found in developing organs or regions as diverse as the gut, the limbs, and the internal and external genitalia. The expression of isolated Hox genes also occurs in locations such as hair follicles, blood cells, and developing sperm cells. The principal function of the Hox genes is involved in setting up structures along the main body axis, but ordered groups of Hox genes are later reused in guiding the formation of several specific nonaxial structures. In mammals, individual members of a paralogous group often have similar functions, so that if one Hox gene is inactivated, the others of that paralogous group may compensate for it. If all members of a paralogous group are inactivated, profound morphological disturbances often result (see p. 171). The Pax gene family, consisting of 9 known members, is an important group of genes that are involved in many aspects of mammalian development (Fig. 4.8). The Pax genes are homologous to the Drosophila pair-rule segmentation genes (see Fig. 4.1). All Pax proteins contain a paired domain of 128 amino acids that binds to DNA. Various members of this group also contain entire or partial homeobox domains and a conserved octapeptide sequence. Pax genes play a variety of important roles in the sense organs and developing nervous system, and outside the nervous system they are involved in cellular differentiative processes when epithelial-mesenchymal transitions occur. The POU gene family is named for the acronym of the first genes identified: Pit1, a gene uniquely expressed in the pituitary; Oct1 and Oct2; and Unc86, a gene expressed in a nematode. Genes of the POU family contain, in addition to a homeobox, a region encoding 75 amino acids, which also bind to DNA through a helix-loop-helix structure. As described in Chapter 3 (see p. 42), Oct-4 plays an important role during early cleavage. The Lim proteins constitute a large family of homeodomain proteins, some of which bind to the DNA in the nucleus and others of which are localized in the cytoplasm. Lim proteins are involved at some stage in the formation of virtually all parts of the body. The absence of certain Lim proteins results in the development of headless mammalian embryos (see p. 83). The transcription factors of the basic helix-loop-helix type are proteins that contain a short stretch of amino acids in which two α-helices are separated by an amino acid loop. This region, with an adjacent basic region, allows the regulatory protein to bind to specific DNA sequences. The basic regions of these proteins bind to DNA, and the helix-loop-helix domain is involved in homodimerization or heterodimerization. This configuration is common in numerous transcription factors that regulate myogenesis (see Fig. 9.33). The zinc finger family of transcription factors consists of proteins with regularly placed cystidine and histidine units that are bound by zinc ions to cause the polypeptide chain to pucker into fingerlike structures (Fig. 4.9). These “fingers” can be inserted into specific regions in the DNA helix. The Sox genes comprise a large family (>20 members) that have in common an HMG (high-mobility group) domain on the protein. This domain is unusual for a transcription factor in that, with a partner protein, it binds to 7 nucleotides on the minor instead of the major groove on the DNA helix and causes a pronounced conformational change in the DNA. Sox proteins were first recognized in 1990, when the SRY gene was shown to be the male-determining factor in sex differentiation (see p. 389), and the name of this group, Sox, was derived from Sry HMG box. One characteristic of Sox proteins is that they work in concert with other transcription factors to influence expression of their target genes (Fig. 4.10). As may be expected from their large number, Sox proteins are expressed by most structures at some stage in their development. The transforming growth factor- β (TGF-β) superfamily consists of numerous molecules that play a wide variety of roles during embryogenesis and postnatal life. The TGF family was named because its first-discovered member (TGF-β1) was isolated from virally transformed cells. Only later was it realized that many signaling molecules with greatly different functions during embryonic and postnatal life bear structural similarity to this molecule. Table 4.1 summarizes some of these molecules and their functions. Table 4.1 Members of the Transforming Growth Factor-β Superfamily Mentioned in This Text
Molecular Basis for Embryonic Development
Fundamental Molecular Processes in Development
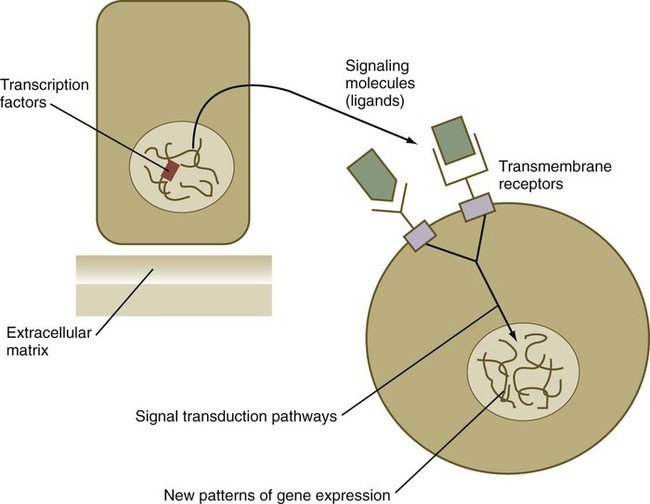
Transcription Factors
Homeobox-Containing Genes and Homeodomain Proteins
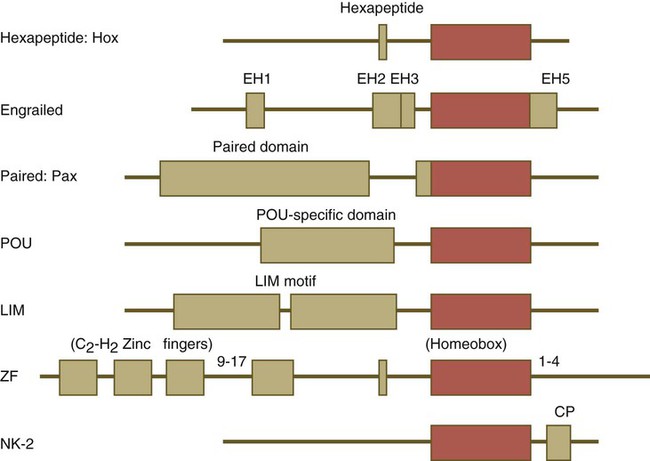
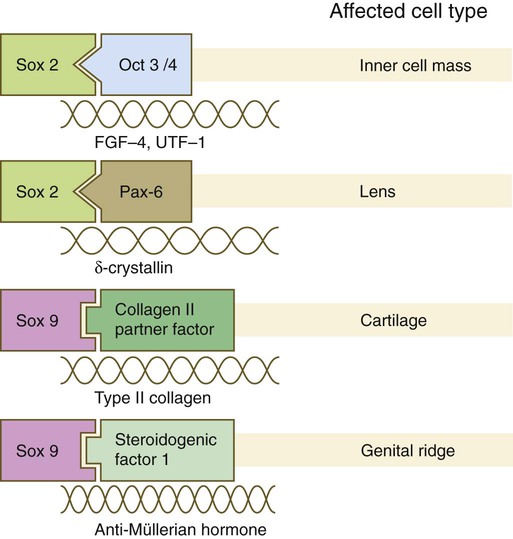
Names of the different classes of genes are listed on the left. The red boxes represent the homeobox within each gene class. The other boxes represent conserved motifs specific to each class of genes. (Modified from Duboule D, ed: Guidebook to the homeobox genes, Oxford, 1994, Oxford University Press.)
HOX Genes
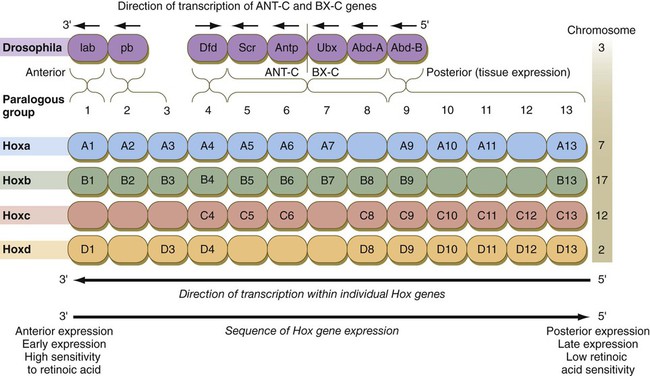
Genes on the 3′ ends of each of the complexes are expressed earlier and more anteriorly than those on the 5′ end (right). (Based on Scott MP: Cell 71:551-553, 1992.)
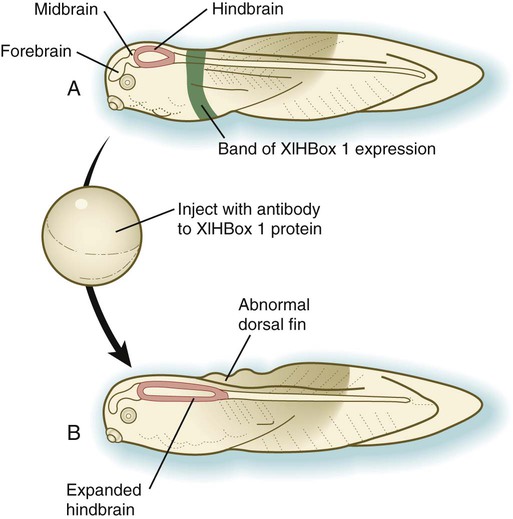
A, Normal larva, showing a discrete band (green) of XlHbox 1 expression. B, Caudal expansion of the hindbrain after antibodies to XlHbox 1 protein are injected into the early embryo. (Based on Wright CV and others: Cell 59:81-93, 1989.)
Pax Genes
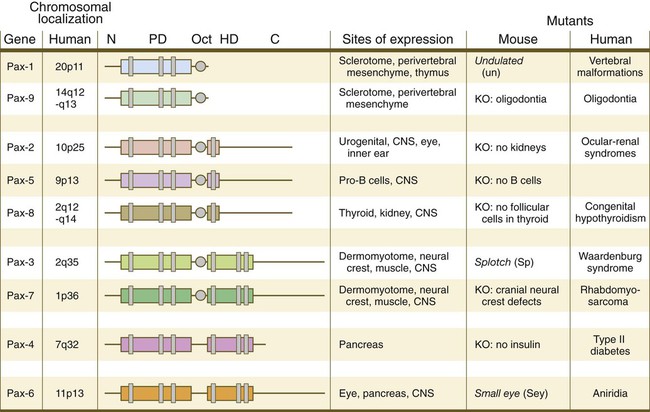
The structures of conserved elements of these genes are schematically represented. CNS, central nervous system; KO, knockout. (Modified from Wehr R, Gruss P: Int J Dev Biol 40:369-377, 1996; and Epstein JC: Trends Cardiovasc Med 6:255-260, 1996.)
Other Homeobox-Containing Gene Families
Helix-Loop-Helix Transcription Factors
Basic Helix-Loop-Helix Proteins
Zinc Finger Transcription Factors
Sox Genes
Signaling Molecules
Transforming Growth Factor-β Family
Member
Representative Functions
Chapters
TGF-β1 to TGF-β5
Mesodermal induction
5
Myoblast proliferation
9
Invasion of cardiac jelly by atrioventricular endothelial cells
17
Activin
Granulosa cell proliferation
1
Mesodermal induction
5
Inhibin
Inhibition of gonadotropin secretion by hypophysis
1
Müllerian inhibiting substance
Regression of paramesonephric ducts
16
Decapentaplegic
Signaling in limb development
10
Vg1
Mesodermal and primitive streak induction
5
BMP-1 to BMP-15
Induction of neural plate, induction of skeletal differentiation, and other inductions
5, 9, 10
Nodal
Formation of mesoderm and primitive streak, left-right axial fixation
5
Glial cell line–derived neurotrophic factor
Induction of outgrowth of ureteric bud, neural colonization of gut
16, 12
Lefty
Determination of body asymmetry
5
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree