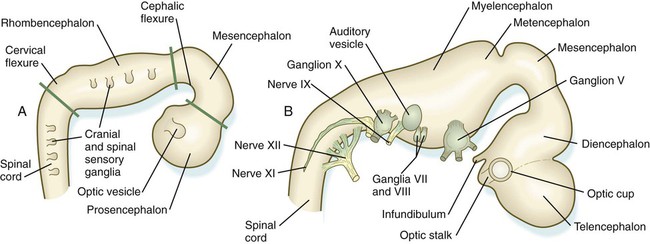
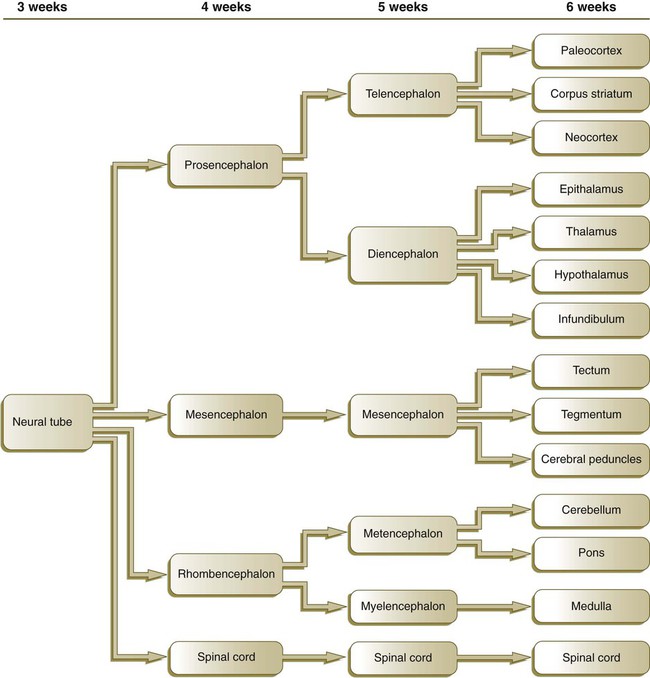
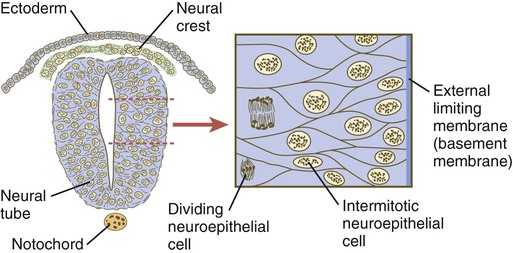
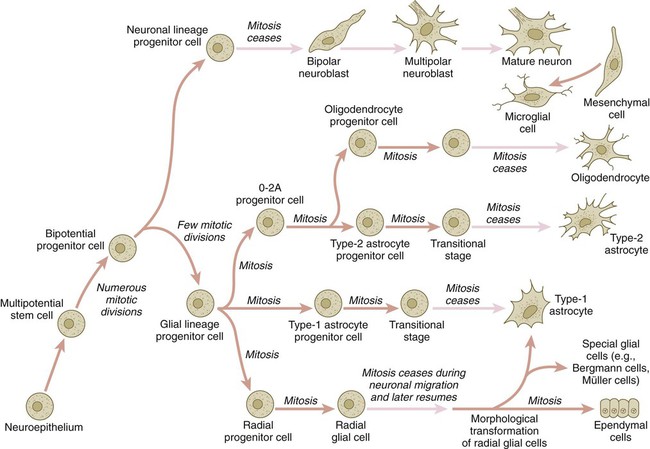
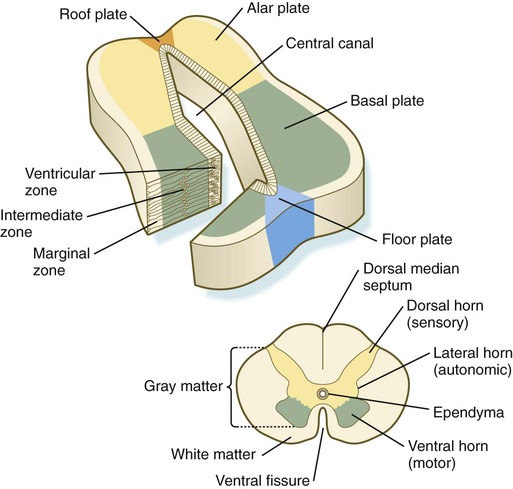
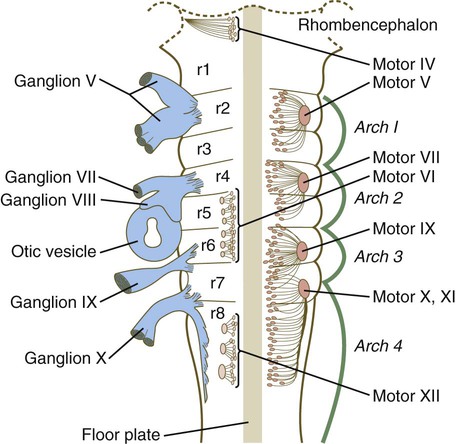
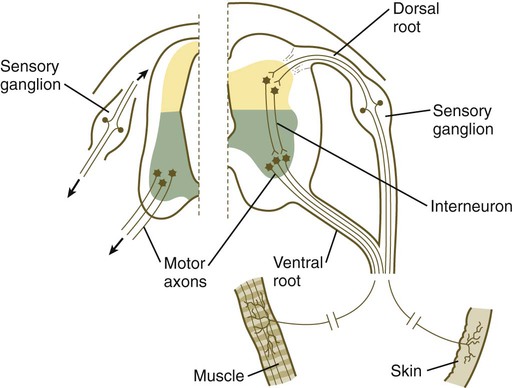
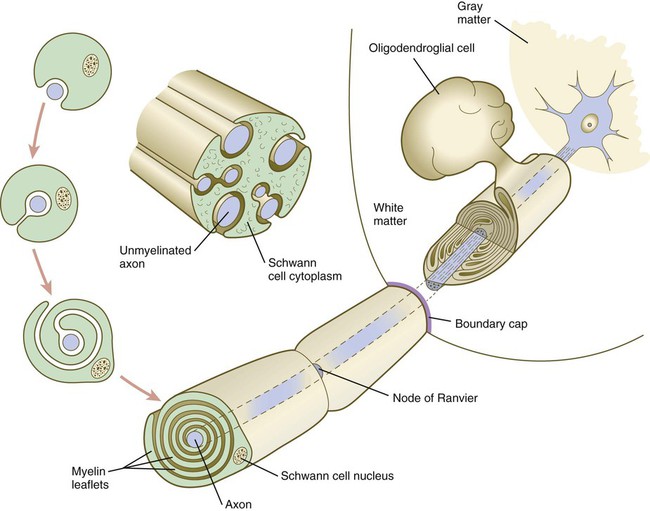
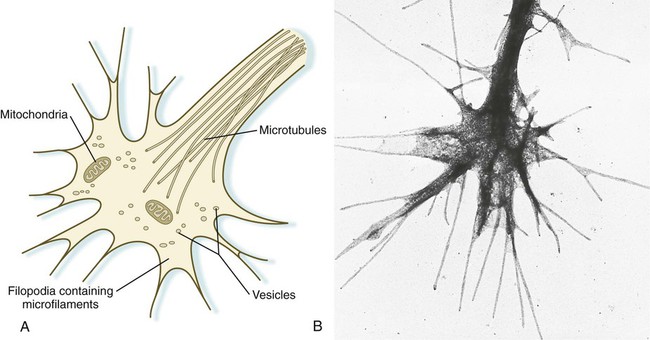
Chapter 11 1. Induction, including primary induction of the nervous system by the underlying notochord and secondary inductions driven by neural tissues themselves 2. Proliferation, first as a response of the neuroectodermal cells to primary induction and later to build up critical numbers of cells for virtually all aspects of morphogenesis of the nervous system 3. Pattern formation, in which cells respond to genetic or environmental cues in forming the fundamental subdivisions of the nervous system 4. Determination of the identity of specific types of neuronal or glial cells 5. Intercellular communication and the adhesion of like cells 6. Cell migration, of which a variety of distinct patterns is found in the nervous system 7. Cellular differentiation of neurons and glial cells 8. Formation of specific connections or synapses between cells 9. Stabilization or elimination of specific interneuronal connections, sometimes associated with massive episodes of cell death of unconnected neurons 10. Progressive development of integrated patterns of neuronal function, which results in coordinated reflex movements As described in Chapter 5, primary induction of the nervous system results in the formation of a thickened ectodermal neural plate overlying the notochord. Much of the dorsal ectoderm in gastrulating embryos produces the signaling protein bone morphogenetic protein-4 (BMP-4), which inhibits the dorsal ectoderm from forming neural tissue. Instead of sending positive signals to the overlying ectoderm, the neural inducers, noggin and chordin, block the inhibitory influence of BMP-4 and allow the dorsal ectoderm to form neural tissue (the neural plate [see Fig. 5.8]). Shortly after neural induction, further signals from the notochord and head organizing regions (prechordal plate and anterior visceral endoderm) result in the expression of the transcription factor Otx-2 in the forebrain-midbrain region and Gbx2 in the hindbrain region. The expression boundary between these two transcription factors forms the isthmic organizer. The signaling molecules fibroblast growth factor-8 (FGF-8) and Wnt-1 diffuse from this boundary and are instrumental in setting up the pattern for forming the midbrain and hindbrain. Then, under the influence of specific combinations of Hox genes and other transcription factors, the hindbrain undergoes a highly regular segmentation into rhombomeres, which presage the overall organization of the entire facial and cervical region (see Fig. 11.12). The neural tube, which is the morphological manifestation of the earliest stages in establishing the nervous system, is a prominent structure. In a human, it dominates the cephalic end of the embryo (see Fig. 6.1). This chapter describes how the early neural tube develops into the major morphological and functional components of the mature nervous system. Closure of the neural tube first occurs in the region where the earliest somites appear; closure spreads cranially and caudally (see Fig. 6.1). The unfused regions of the neural tube are known as the cranial and caudal neuropores. Even before the closure of the neuropores (24 days’ gestation for the cranial neuropore and 26 days’ gestation for the caudal neuropore), some fundamental subdivisions in the early nervous system have become manifest. The future spinal cord and brain are recognizable, and within the brain the forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon) can be distinguished (Fig. 11.1A). A prominent force in shaping the early nervous system is the overall bending of the cephalic end of the embryo into a “C” shape. Associated with this bending is the appearance at the end of the third week of a prominent cephalic flexure of the brain at the level of the mesencephalon (see Fig. 11.1A). Soon the brain almost doubles back on itself at the cephalic flexure. At the beginning of the fifth week, a cervical flexure appears at the boundary between the hindbrain and the spinal cord. By the fifth week, the original three-part brain has become subdivided further into five parts (Fig. 11.2; see Fig. 11.1B). The prosencephalon gives rise to the telencephalon (endbrain), with prominent lateral outpocketings that ultimately form the cerebral hemispheres, and a more caudal diencephalon. The diencephalon is readily recognizable because of the prominent lateral optic vesicles that extend from its lateral walls. The mesencephalon, which is sharply bent by the cephalic flexure, remains undivided and tubular in its overall structure. The roof of the rhombencephalon becomes very thin, and there are early indications of the subdivision of the rhombencephalon into a metencephalon and a more caudal myelencephalon. These five subdivisions of the early brain represent a fundamental organization that persists through adulthood. Many further structural and functional components give added layers of complexity to the brain over the next several weeks of embryonic life. Shortly after induction, the thickening neural plate and early neural tube become organized into a pseudostratified epithelium (Fig. 11.3). In this type of epithelium, the nuclei appear to be located in several separate layers within the elongated neuroepithelial cells. The nuclei undertake extensive shifts of position within the cytoplasm of the neuroepithelial cells. The neuroepithelial cells are characterized by a high degree of mitotic activity, and the position of the nuclei within the neural tube and their stage in the mitotic cycle are closely correlated (Fig. 11.4). DNA synthesis occurs in nuclei located near the external limiting membrane (the basal lamina surrounding the neural tube). As these nuclei prepare to go into mitosis, they migrate within the cytoplasm toward the lumen of the neural tube, where they undergo mitotic division. The orientation of the mitotic spindle during this division predicts the fate of the daughter cells. If the metaphase plate (plane of cleavage) is perpendicular to the apical (inner) surface of the neural tube, the two daughter cells slowly migrate in tandem back toward the outer side of the neural tube, where they prepare for another round of DNA synthesis (see Fig. 11.4). In contrast, if the plane of cleavage is parallel to the inner surface of the neural tube, the daughter cells undergo dramatically different fates. The daughter cell that is closer to the inner surface migrates away very slowly and remains a proliferative progenitor cell that is capable of mitosis. The daughter cell that is closer to the basal surface (external limiting membrane) inherits a high concentration of the Notch receptor on its surface and quickly moves away from the apical surface as a postmitotic neuroblast (see Fig. 11.4). The neuroblasts, cellular precursors of neurons, begin to produce cell processes that ultimately become axons and dendrites. The origins of most cells found in the mature central nervous system can be traced to multipotential stem cells within the early neuroepithelium (Fig. 11.5). These cells undergo numerous mitotic divisions before maturing into bipotential progenitor cells, which give rise to either neuronal or glial progenitor cells. Activation of the proneural genes neurogenin 1 and neurogenin 2 promotes the differentiation of neurons from the bipolar progenitor cells. Glial cells differentiate under the influence of other stimuli. This developmental bifurcation is accompanied by a change in gene expression. Multipotential stem cells express an intermediate filament protein called nestin. Nestin is downregulated as descendants of bipolar progenitor cells separate into neuronal progenitor cells, which express neurofilament protein, and glial progenitor cells, which express glial fibrillary acidic protein. The other major lineage stemming from the bipotential progenitor cells is the glial line. Glial progenitor cells continue to undergo mitosis, and their progeny split into several lines. One, the O-2A progenitor cell (see Fig. 11.5), is a precursor to two lines of glial cells that ultimately form the oligodendrocytes and type 2 astrocytes. Another glial lineage gives rise to type 1 astrocytes. Human oligodendrocytes arise from progenitor cells located in the ventral ventricular zone (see MN in Fig. 11.10) alongside the floor plate. From there, they spread throughout the brain and spinal cord and ultimately form the myelin coverings around neuronal processes in the white matter. The formation of oligodendrocyte precursors depends on an inductive signal from the notochord (sonic hedgehog [shh]). If the notochord is transplanted alongside dorsal neural tube, oligodendrocyte precursors differentiate there, thus showing that cells with the potential to form oligodendrocytes reside in that area, but normally do not develop because of the lack of an adequate inductive signal. The third glial lineage has a more complex history. Radial progenitor cells give rise to radial glial cells, which act as guidewires in the brain for the migration of young neurons (see Fig. 11.23). When the neurons are migrating along the radial glial cells during midpregnancy, they inhibit the proliferation of the radial glial cells. After neuronal cell migration, the radial glial cells, now free from the inhibitory influence of the neurons, reenter the mitotic cycle. Their progeny can transform into several cell types. Some can seemingly cross lineage lines and differentiate into type 1 astrocytes (see Fig. 11.5). Other progeny differentiate into various specialized glial cell types, ependymal cells, and even adult neural stem cells. According to some authors, the remaining neuroepithelial cells represent another source of ependymal cells. The developing spinal cord is a useful prototype for studying the overall structural and functional features of the central nervous system because it preserves its fundamental organization through much of development. With the beginning of cellular differentiation in the neural tube, the neuroepithelium thickens and appears layered. The layer of cells closest to the lumen (central canal) of the neural tube remains epithelial and is called the ventricular zone (the ependymal zone in older literature). This zone, which still contains mitotic cells, ultimately becomes the ependyma, a columnar epithelium that lines the ventricular system and central canal of the central nervous system (Fig. 11.6). Farther from the ventricular zone is the intermediate (formerly called mantle) zone, which contains the cell bodies of the differentiating postmitotic neuroblasts. As the neuroblasts continue to produce axonal and dendritic processes, the processes form a peripheral marginal zone that contains neuronal processes, but not neuronal cell bodies. As the spinal cord matures, the intermediate zone becomes the gray matter, in which the cell bodies of the neurons are located. The marginal zone is called the white matter because of the color imparted by the numerous tracts of myelinated nerve fibers in that layer (see Fig. 11.6). During development, the proliferating progenitor cell populations in the ventricular zone become largely exhausted, but it is now known that a subpopulation persists into adulthood as neural stem cells. The remaining cells differentiate into the epithelium of the ependymal layer. The basal plate represents the motor component of the spinal cord. Axons arising from neurons located in the ventral horn of the gray matter exit the spinal cord as ventral motor roots of the spinal nerves (see Fig. 11.15). The gray matter of the alar plate, called the dorsal horn, is associated with sensory functions. Sensory axons from the spinal ganglia (neural crest derivatives) enter the spinal cord as dorsal roots and synapse with neurons in the dorsal horn. A small projection of gray matter between the dorsal and ventral horns at spinal levels T1 to L2 contains cell bodies of autonomic neurons. This projection is called the lateral horn or sometimes the intermediolateral gray column (see Fig. 11.6). The floor plate is far more than an anatomical connection between the right and left basal plates. Cells of the future floor plate are the first to differentiate in the neural plate after primary induction of the nervous system. Experimental work has shown a specific inductive influence of the notochord on the neuroepithelial cells that overly it. If an extra notochord is grafted along the lateral surface of the neural tube, the neuroepithelial cells closest to it acquire the properties of floor plate cells (Fig. 11.7). Conversely, if a segment of normal notochord is removed, the neuroepithelial cells overlying it do not acquire the properties of floor plate cells. Through its action on the floor plate, the notochord also exerts a profound effect on the organization of the dorsal and ventral roots that enter and leave the spinal cord. If the notochord is absent, the neural tube closes, but recognizable dorsal and ventral roots are absent. Numerous ectopic nerve fibers appear in their place (Fig. 11.8). If the future floor plate is split, the side of the neural tube on which the notochord is located develops normal dorsal and ventral roots, whereas the side lacking these structures gives off ectopic nerves (see Fig. 11.7C). A molecular basis for cross-sectional pattern formation within the early neural plate and neural tube has been identified (Fig. 11.9). The homeobox-containing transcription factors, Pax-3, Pax-7, Msx-1, and Msx-2, are expressed throughout the early neural plate. Before the neural plate has folded over to become the neural tube, the notochord, which is adherent to the midline neural plate at this stage, releases shh. Local hedgehog signaling stimulates the neural plate cells directly above the notochord to transform into the floor plate. One of the first stages of this transformation is the repression of Pax-3 and Pax-7 expression, which allows the neuroectodermal cells near the midline of the neural plate to adopt a ventral fate (i.e., floor plate or basal plate). Cells of the floor plate itself then become sites of production of shh. Development of the overall cross-sectional organization of the neural tube involves not only a ventralizing influence from the notochord, but also an opposing dorsalizing influence from the epidermal ectoderm adjacent to the developing neural tube. In the lateral regions of the neural plate (future dorsal region of the neural tube), BMP-4 and BMP-7, expressed by non-neural ectoderm at the ectodermal–lateral neural plate junction, exert a dorsalizing inductive effect on the neuroectodermal cells that results in the formation of the roof plate, which takes shape soon after the last neural crest cells have emigrated from the neural tube. BMP within the roof plate acts as a patterning signal and induces the further dorsalizing molecules Pax-3, Pax-7, Msx-1, and Msx-2 (see Fig. 11.9). Dorsal Wnt signaling promotes the proliferation of neural progenitor cells and also works with BMPs as an overall dorsalizing influence in the dorsoventral patterning of neurons. After closure of the neural tube, signals from the roof plate induce a series of six early and two late generated dorsal interneuronal types in a manner reminiscent of the better defined specification of ventral interneurons (see later). While the broader regions of the cross section of the spinal cord are being set in place, a tightly controlled molecular grid forms the basis for specification of the major types of neurons found in the ventral part of the spinal cord. Within the basal plate is an array of five types of neurons—motoneurons and four types of interneurons—that are arranged in a well-defined dorsoventral pattern. These classes of neurons are specified by specific combinations of homeodomain transcription factors, whose pattern of expression is set by a gradient of shh emanating from the floor plate as modulated by the activating and repressive properties of the Gl-1 to Gl-3 proteins (Fig. 11.10). Some of these transcription factors (class I) are repressed at various dorsoventral levels by the shh gradient, whereas others (class II) are induced by shh (see Fig. 11.10). The net result is that a different combination of the transcription factors at each dorsoventral level specifies each of the five types of neurons, which are characterized by their own unique molecular signature. One in particular, islet-1, is characteristic of motoneurons. Shortly after the production of motoneurons has ceased, a shift in regulatory factors stimulates the production of glial progenitor cells from the ventral neuroepithelium. This leads to the formation of the oligodendrocytes that become closely associated with the neurons. Through neural induction, the early central nervous system becomes organized into broad regions that develop cranial, middle, and caudal characteristics. This is soon followed by the appearance of the morphological subdivisions outlined in Figure 11.2. At an even finer level, segments called rhombomeres appear in the region of the hindbrain (see Fig. 6.3), and a less distinct series of subdivisions called prosomeres appears in the forebrain. The rhombomeres (Fig. 11.11), introduced in Chapter 6, are the morphological reflection of a highly segmentally ordered pattern of expression of a variety of developmentally prominent transcription factors (Fig. 11.12). The establishment of the isthmic organizer and the pathways that set up this pattern are discussed in Chapter 6 (see pp. 95-96). The correspondence between the rhombomeres of the developing hindbrain and other structures of the cranial and pharyngeal arch region is remarkable (see Chapter 14). The cranial nerves, which have a highly ordered pattern by which they supply structures derived from the pharyngeal arches and other structures in the head, have an equally highly ordered origin with respect to the rhombomeres (Fig. 11.13). Cranial nerve V innervates structures derived from the first pharyngeal arch. Cranial nerves VII and IX innervate the second and third arch structures. In embryos of birds, the species studied most extensively, cell bodies of the motor components of cranial nerves V, VII, and IX are initially found exclusively in rhombomeres 2, 4, and 6. The cell bodies (within the central nervous system, the collection of cell bodies of a single cranial nerve is called a nucleus) of the cranial nerves that innervate the pharyngeal arches arise in register along the craniocaudal axis. The motor nuclei of other cranial nerves that innervate somatic structures (e.g., extraocular muscles or the tongue) arise in a different craniocaudal column along the hindbrain and do not occupy contiguous rhombomeres (see Fig. 11.13). A fundamental patterning mechanism in the midbrain region is a molecular signaling center (isthmic organizer) located at the border between the mesencephalon and the metencephalon (see Fig. 6.4). The principal signaling molecule is FGF-8, which is expressed in a narrow ring at the anterior border of the first rhombomere, a subdivision of the metencephalon. Acting with Wnt-1, FGF-8 induces the expression of engrailed genes En-1 and En-2 and Pax-2 and Pax-5, which are expressed in decreasing concentrations at increasing distances from the FGF-8 signaling center (see Fig. 6.4). The main function of Wnt-1 seems to be the stimulation of local cell proliferation, whereas the overall organizing function belongs to FGF-8. The isthmic organizer induces and polarizes the dorsal midbrain region and the cerebellum. Grafts of the isthmic organizer or beads releasing FGF-8 alone into more cranial regions of the forebrain of the avian embryo induce a second tectum (dorsal mesencephalon or colliculi in mammals). Similarly, isthmic grafts into regions of the hindbrain can induce supernumerary cerebellar structures. Cranially, the midbrain is separated from the forebrain (diencephalon) through a different set of molecular interactions. The alar plate of the diencephalon is characterized by the expression of Pax-6, a molecule that, among other things, acts as the master gene underlying eye formation (see Chapter 13). The mesencephalon is a domain of En-1 expression. Through the action of intermediate negative regulators, Pax-6 inhibits En-1 expression, whereas En-1 directly inhibits Pax-6 expression. The craniocaudal level at which each of these molecules inhibits the other becomes a sharp diencephalic-mesencephalic border. Although much less apparent than in the hindbrain, the neuromeric organization of the early brain extends into the forebrain region as a set of three prosomeres, which extend from the midbrain-forebrain boundary through the thalamus (Fig. 11.14). Prosomeres 1 to 3 (p1 to p3), the most posterior prosomeres, become incorporated into the diencephalon, with p2 and p3 forming the dorsal and ventral thalamus, which serves as a major relay station for transmitting neural signals between the cerebral cortex and the body. An earlier representation suggested that an additional set of prosomeres (p4 to p6) formed the organizational basis for the rostral diencephalon (hypothalamus) and telencephalon. A more recent interpretation, however, places these structures in what has been called the secondary rhombencephalon, a developmental field that encompasses the entire prechordal portion of the neural tube. Within this domain, the basal plate develops into the major regions of the hypothalamus, the structure that integrates autonomic nervous functions and controls endocrine release from the pituitary. The alar plate in this domain is the precursor of the cerebral cortex, the basal ganglia (collectively the telencephalon), and the optic vesicles (diencephalic structures that take the lead in formation of the eyes). As development progresses, the secondary rhombencephalon becomes sharply folded beneath p2 to p3, and in humans the enormous outgrowth of the alar plates of the secondary rhombencephalon envelops the other prosomeres as the telencephalic vesicles (future cerebral cortex). Discrete patterns of gene expression also mirror the basic regional organization of the forebrain. Early in development, FGF-8, secreted by the anterior neural ridge (see Fig. 6.4B), induces the expression of Foxg-1, formerly called BF-1 (brain factor-1), which regulates development of the telencephalon and optic vesicles. Within the forebrain, a thin rim of expression of the transcription factor Nkx 2.2 marks the border between alar and basal plates. Along the dorsoventral axis, the alar plates are characterized by the expression of Emx1 and Emx2 and Pax6, important regulators of regional identity within the cerebral cortex. In an intermediate region of the prosencephalic alar plate, Emx expression drops out, leaving Pax-6 to function as the master gene controlling eye development (see Chapter 13). In the more ventral area that eventually forms the basal ganglia, Dlx (distalless) is a dominant expressed gene. Similar to the spinal cord, the ventral forebrain is induced and organized by shh, secreted by midline axial structures. In the absence of shh signaling in this area, the tissues of the ventral forebrain are greatly reduced, leading sometimes to midline fusion of the optic vesicles and a general reduction of the growth of the midface region. This situation results in a type of anomaly called holoprosencephaly (see p. 309), which in extreme cases is accompanied by cyclopia. At the border between the future dorsal (p2) and ventral (p3) thalamus is a narrow band of shh expression called the zona limitans interthalamica. Signals emanating from the zona limitans interthalamica specify aspects of cellular identity and behavior in the diencephalic regions on either side (see Fig. 6.4B). The mechanisms leading to the formation of the zona limitans interthalamica are poorly understood, but this structure arises directly above the anterior tip of the notochord. This finding suggests that the formation of this structure, similar to that of the isthmus, is a reflection of an earlier molecular boundary zone. The formation of a peripheral nerve begins with the outgrowth of axons from motor neuroblasts located in the basal plate (the future ventral horn of the gray matter) of the spinal cord (Fig. 11.15). Near the dorsal part of the spinal cord, thin processes also begin to grow from neural crest–derived neuroblasts that have aggregated to form the spinal ganglia. Dendrites, which conduct impulses toward the nerve cell body, grow from the sensory neurons toward the periphery. Axons, which conduct impulses away from the cell body, penetrate the dorsolateral aspect of the spinal cord and terminate in the dorsal horn (the gray matter of the alar plate). Within the gray matter, short interneurons connect the terminations of the sensory axons to the motoneurons. These three connected neurons (motor, sensory, and interneuron) constitute a simple reflex arc through which a sensory stimulus can be translated into a simple motor response. Autonomic nerve fibers are also associated with typical spinal nerves. A long-standing question concerning the organization of the nervous system involves the interface between the central nervous system and the peripheral nervous system, in particular how their respective cellular components are kept separate. Such separation at the points of exit of the motor axons from the ventral neural tube and the entry into the dorsal neural tube of spinal axons from the dorsal roots is accomplished by localized boundary caps of neural crest cells (Fig. 11.16). The boundary caps act as selective filters, which allow the free passage of outgrowing and ingrowing axons between the neural tube and the periphery, but which serve as a barrier to keep cells in their appropriate compartment. In the absence of boundary caps, many cell bodies of motoneurons translocate away from the lateral motor column (their normal location) into the space outside the neural tube. Within a peripheral nerve, the neuronal processes can be myelinated or unmyelinated. At the cellular level, myelin is a multilayered spiral sheath consisting largely of phospholipid material that is formed by individual Schwann cells (neural crest derivatives) that wrap themselves many times around a nerve process like a jelly roll (see Fig. 11.16). This wrapping serves as a form of insulation that largely determines the character of the electrical impulse (action potential) traveling along the neuronal process. Unmyelinated nerve fibers are also embedded in the cytoplasm of Schwann cells, but they lack the characteristic spiral profiles of myelinated processes (see Fig. 11.16). An actively elongating neurite is capped by a growth cone (Fig. 11.17). Growth cones are characterized by an expanded region of cytoplasm with numerous spikelike projections called filopodia. In vitro and in vivo studies of living nerves show that the morphology of an active growth cone is in a constant state of flux, with filopodia regularly extending and retracting as if testing the local environment. Growth cones contain numerous cytoplasmic organelles, but much of the form and function of the filopodia depends on the large quantities of actin microfilaments that fill these processes. In a growth cone, an equilibrium exists between the extension of actin microfilaments by terminal addition and resorption at the proximal end. Under conditions favorable for growth, the balance tips toward extension, whereas an unfavorable environment results in the resorption of the microfilaments and collapse of the growth cone. Many molecules are involved in the guidance of neuronal outgrowth (Table 11.1). Some of these molecules can exert either attractive or repulsive actions, depending on the character of the neuron, the time in development, or some combination of intrinsic and extrinsic characteristics. To a greater or lesser extent, the nature of the reaction of the growth cone is determined locally, because in vitro studies have shown that such reactions can occur even when the neuronal process is cut off from the cell body. Table 11.1 Receptor/Ligand Pairs Known to Influence Axonal Outgrowth* During Embryonic Development
Nervous System
Establishment of the Nervous System
Early Shaping of the Nervous System
Histogenesis Within the Central Nervous System
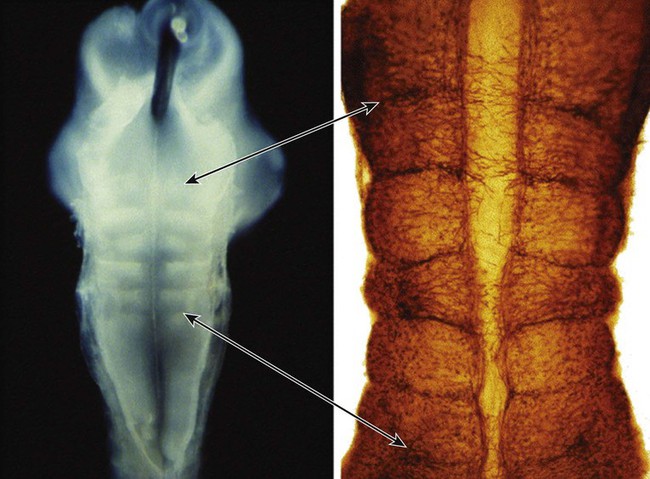
Proliferation Within the Neural Tube
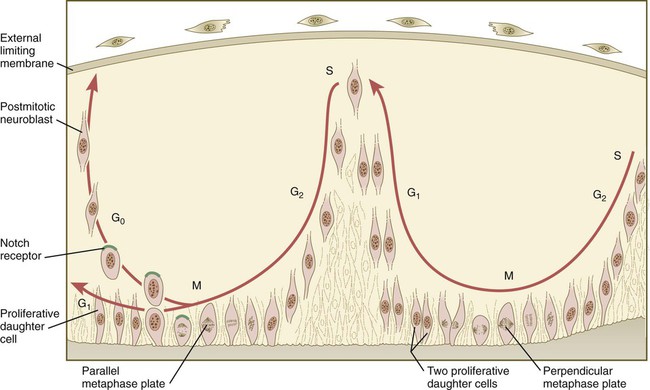
In the pseudostratified epithelial cells that constitute the early neural tube, nuclei that synthesize DNA (S phase) are located near the external limiting membrane. These nuclei move toward the inner margin of the neural tube, where mitosis (M) occurs. If the metaphase plate is perpendicular to the inner margin, the two daughter cells remain in a proliferative state and migrate back toward the external limiting membrane for another round of DNA synthesis. If in the next mitosis the metaphase plate is oriented parallel to the inner margin, one daughter cell remains in the proliferative state. The other daughter cell, which expresses Notch, leaves the mitotic cycle to become a neuroblast.
Cell Lineages in Histogenesis of the Central Nervous System
Fundamental Cross-Sectional Organization of the Developing Neural Tube
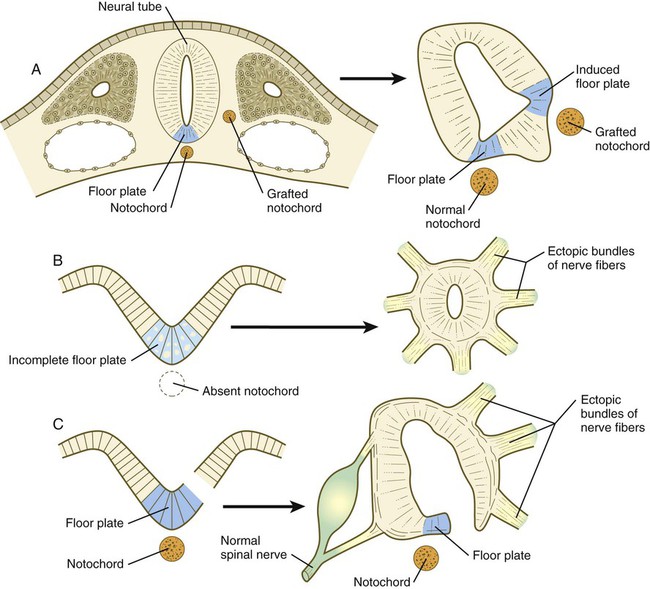
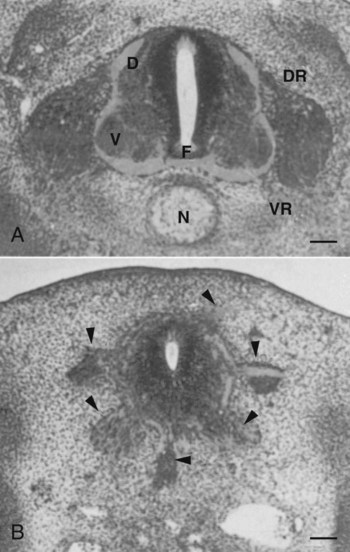
A, Grafting an extra notochord near the neural tube induces a secondary floor plate. B, In the absence of a notochord, a very incomplete floor plate forms, and nerve fibers exit from multiple sites around the spinal cord. C, Slitting the neural plate on one side of the floor plate removes the wall of the neural tube from the influence of the notochord, allowing the disorganized exit of nerve fibers from that part of the spinal cord. (Adapted from Hirano S, Fuse S, Sohal GS: Science 251:310-313, 1991.)
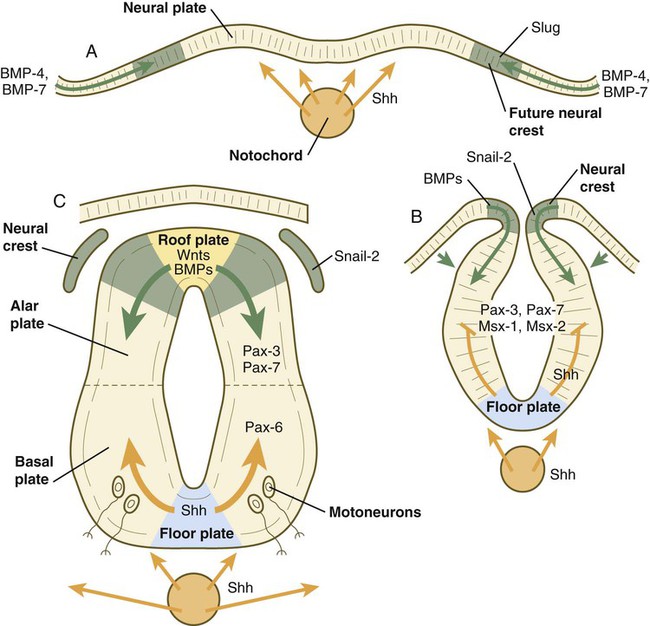
A, Signals from sonic hedgehog (Shh) (orange arrows) in the notochord induce the floor plate. B, In the dorsal part of the future neural tube, bone morphogenetic protein-4 (BMP-4) and BMP-7 (green arrows) from the ectoderm adjacent to the neural tube induce snail-2 in the future neural crest and maintain Pax-3 and Pax-7 expression dorsally. Ventrally, Shh, now produced by the floor plate, induces motoneurons. C, Shh, produced by the floor plate, suppresses the expression of dorsal Pax genes (Pax-3 and Pax-7) in the ventral half of the neural tube. Wnts and BMPs counter this effect by exerting a dorsalizing influence.
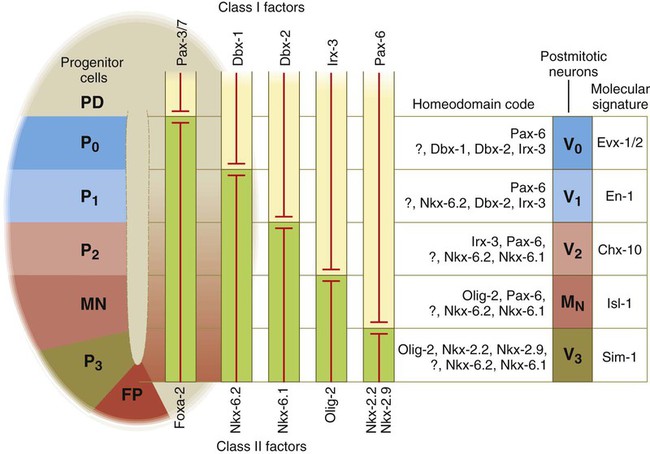
At the left, various classes of progenitor neurons are given labels beginning in P. At the right of the neural tube, a decreasing concentration gradient of sonic hedgehog is indicated by the fading red-brown background. The class I factors (upper bars) are repressed by sonic hedgehog, whereas the class II factors are induced by sonic hedgehog. To the right of the bars is the set of homeodomain codes that specify the various levels of precursor neurons, and at the far right is the molecular signature of the neurons. FP, floor plate; MN, motoneuron precursors.
Craniocaudal Pattern Formation and Segmentation
Patterning in the Hindbrain and Spinal Cord Regions
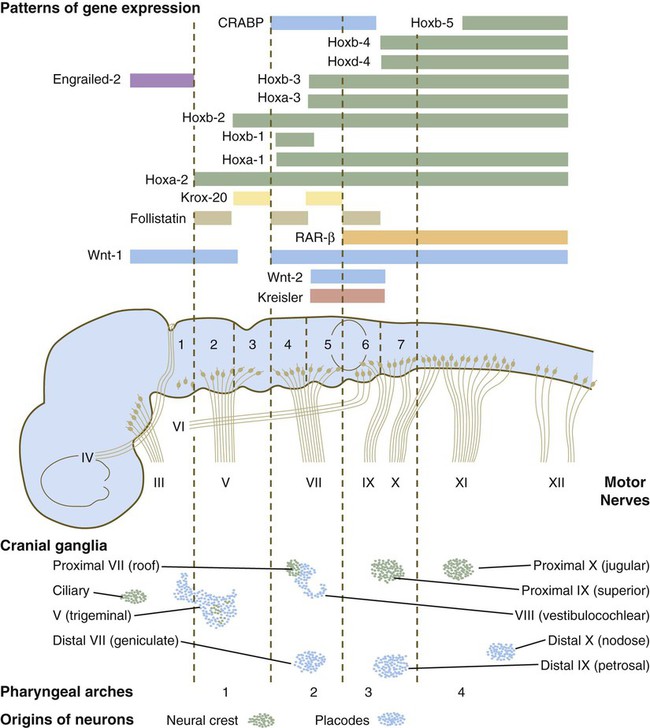
The bars refer to craniocaudal levels of expression of a given gene product. Cranial sensory nerves derived from the neural crest and placodal precursors are laid out in proper register. CRABP, cytoplasmic retinoic acid–binding protein; RAR, retinoic acid receptor. (Adapted from Noden DM: J Craniofac Genet Dev Biol 11:192-213, 1991.)
Patterning in the Midbrain Region
Patterning in the Forebrain Region
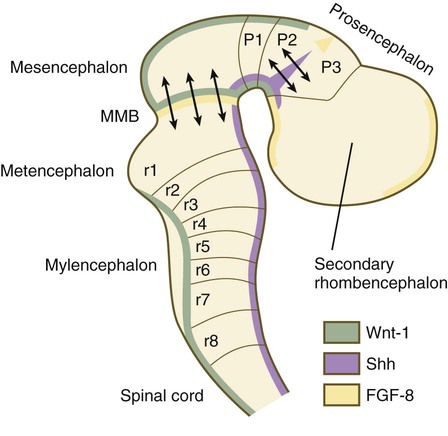
The midbrain/hindbrain signaling region is indicated by the arrows just rostral to the first rhombomere. The arrows between the second and third prosomeres represent the zona limitans interthalamica, a signaling region in the forebrain. MMB, mesencephalic-metencephalic border. (Adapted from Bally-Cuif L, Wassef M: Determination events in the nervous system of the vertebrate embryo, Curr Opin Genet Dev 5:450-458, 1995.)
Peripheral Nervous System
Structural Organization of a Peripheral Nerve
Patterns and Mechanisms of Neurite Outgrowth
Ligand
Receptor
Slit
Robo-1, Robo-4
Ephrin
Eph
Netrin
UNC-5, DCC
Semaphorin
Plexin, Neuropilin
VEGF
VEGFR, Neuropilin
Draxin
Netrin receptors
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nervous System