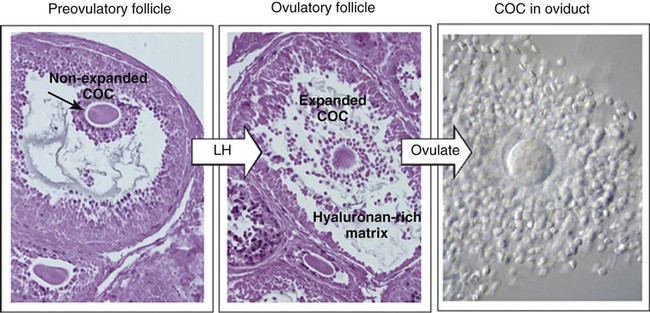
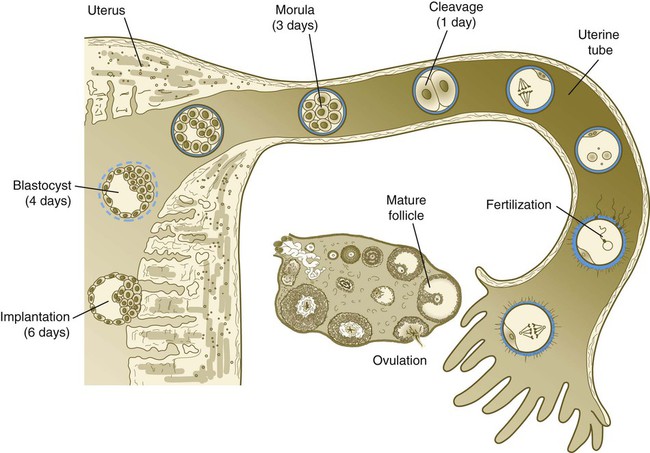
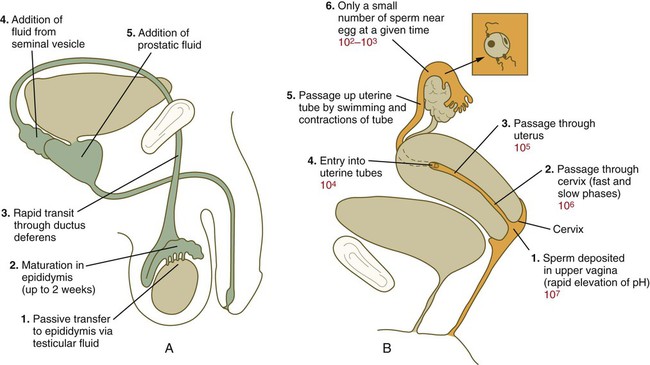
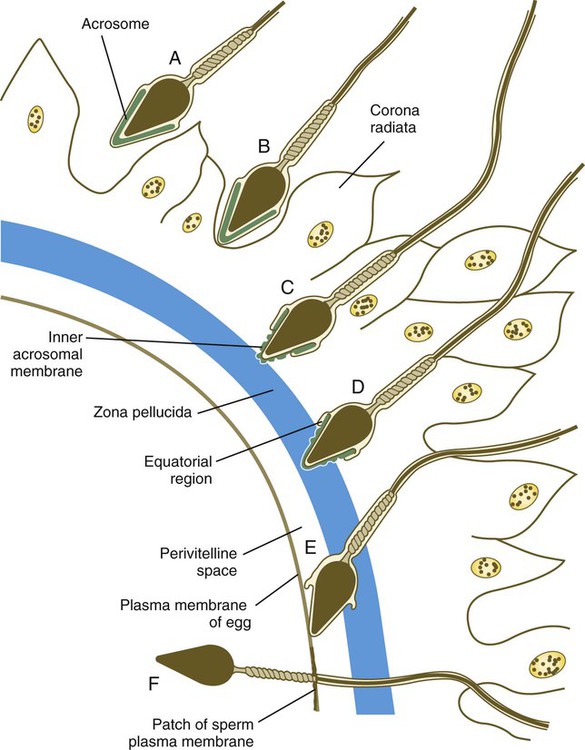
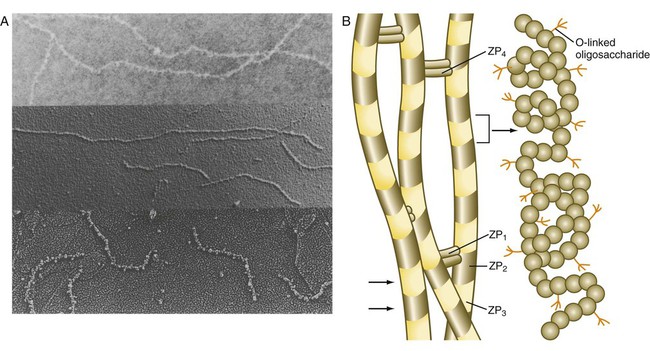
Chapter 2 Chapter 1 describes the origins and maturation of male and female gametes and the hormonal conditions that make such maturation possible. It also describes the cyclic, hormonally controlled changes in the female reproductive tract that ready it for fertilization and the support of embryonic development. This chapter first explains the way the egg and sperm cells come together in the female reproductive tract so that fertilization can occur. It then outlines the complex set of interactions involved in fertilization of the egg by a sperm. The stimulus for ovulation is the surge of LH secreted by the anterior pituitary at the midpoint of the menstrual cycle (see Fig. 1.16). Within hours of exposure to the LH surge, the follicle reorganizes its program of gene expression from one directed toward development of the follicle to one producing molecules that set into gear the processes of follicular rupture and ovulation. Shortly after the LH peak, local blood flow increases in the outer layers of the follicular wall. Along with the increased blood flow, plasma proteins leak into the tissues through the postcapillary venules, with resulting local edema. The edema and the release of certain pharmacologically active compounds, such as prostaglandins, histamine, vasopressin, and plasminogen activator, provide the starting point for a series of reactions that result in the local production of matrix metalloproteinases—a family of lytic enzymes that degrade components of the extracellular matrix. At the same time, the secretion of hyaluronic acid by cells of the cumulus results in a loosening of the cells surrounding the egg. The lytic action of the matrix metalloproteinases produces an inflammatorylike reaction that ultimately results in rupture of the outer follicular wall about 28 to 36 hours after the LH surge (Fig. 2.1). Within minutes after rupture of the follicular wall, the cumulus oophorus detaches from the granulosa, and the egg is released from the ovary. Tubal transport of the egg usually takes 3 to 4 days, whether or not fertilization occurs (Fig. 2.2). Egg transport typically occurs in two phases: slow transport in the ampulla (approximately 72 hours) and a more rapid phase (8 hours) during which the egg or embryo passes through the isthmus and into the uterus (see p. 51). By a poorly understood mechanism, possibly local edema or reduced muscular activity, the egg is temporarily prevented from entering the isthmic portion of the tube, but under the influence of progesterone, the uterotubal junction relaxes and permits entry of the ovum. By roughly 80 hours after ovulation, the ovulated egg or embryo has passed from the uterine tube into the uterus. If fertilization has not occurred, the egg degenerates and is phagocytized. (Implantation of the embryo is discussed in Chapter 3.) After spermiogenesis in the seminiferous tubules, the spermatozoa are morphologically mature but are nonmotile and incapable of fertilizing an egg (Fig. 2.3). Spermatozoa are passively transported via testicular fluid from the seminiferous tubules to the caput (head) of the epididymis through the rete testis and the efferent ductules. They are propelled by fluid pressure generated in the seminiferous tubules and are assisted by smooth muscle contractions and ciliary currents in the efferent ductules. Spermatozoa spend about 12 days in the highly convoluted duct of the epididymis, which measures 6 m in the human, during which time they undergo biochemical maturation. This period of maturation is associated with changes in the glycoproteins in the plasma membrane of the sperm head. By the time the spermatozoa have reached the cauda (tail) of the epididymis, they are capable of fertilizing an egg. In the female reproductive tract, sperm transport begins in the upper vagina and ends in the ampulla of the uterine tube, where the spermatozoa make contact with the ovulated egg. During copulation, the seminal fluid is normally deposited in the upper vagina (see Fig. 2.3), where its composition and buffering capacity immediately protect the spermatozoa from the acid fluid found in the upper vaginal area. The acidic vaginal fluid normally serves a bactericidal function in protecting the cervical canal from pathogenic organisms. Within about 10 seconds, the pH of the upper vagina is increased from 4.3 to as much as 7.2. The buffering effect lasts only a few minutes in humans, but it provides enough time for the spermatozoa to approach the cervix in an environment (pH 6.0 to 6.5) optimal for sperm motility. Once inside the uterine tube, the spermatozoa collect in the isthmus and bind to the epithelium for about 24 hours. During this time, they are influenced by secretions of the tube to undergo the capacitation reaction. One phase of capacitation is removal of cholesterol from the surface of the sperm. Cholesterol is a component of semen and acts to inhibit premature capacitation. The next phase of capacitation consists of removal of many of the glycoproteins that were deposited on the surface of the spermatozoa during their tenure in the epididymis. Capacitation is required for spermatozoa to be able to fertilize an egg (specifically, to undergo the acrosome reaction; see p. 29). After the capacitation reaction, the spermatozoa undergo a period of hyperactivity and detach from the tubal epithelium. Hyperactivation helps the spermatozoa to break free of the bonds that held them to the tubal epithelium. It also assists the sperm in penetrating isthmic mucus, as well as the corona radiata and the zona pellucida, which surround the ovum. Only small numbers of sperm are released at a given time. This may reduce the chances of polyspermy (see p. 31). While the ovulated egg is passing through the uterine tubes, the ruptured follicle from which it arose undergoes a series of striking changes that are essential for the progression of events leading to and supporting pregnancy (see Fig. 1.8). Soon after ovulation, the basement membrane that separates the granulosa cells from the theca interna breaks down, thus allowing thecal blood vessels to grow into the cavity of the ruptured follicle. The granulosa cells simultaneously undergo a series of major changes in form and function (luteinization). Within 30 to 40 hours of the LH surge, these cells, now called granulosa lutein cells, begin secreting increasing amounts of progesterone along with some estrogen. This pattern of secretion provides the hormonal basis for the changes in the female reproductive tissues during the last half of the menstrual cycle. During this period, the follicle continues to enlarge. Because of its yellow color, it is known as the corpus luteum. The granulosa lutein cells are terminally differentiated. They have stopped dividing, but they continue to secrete progesterone for 10 days. When the spermatozoa first encounter the ovulated egg in the ampullary part of the uterine tube, they are confronted by the corona radiata and some remnants of the cumulus oophorus, which represents the outer layer of the egg complex (Fig. 2.4). The corona radiata is a highly cellular layer with an intercellular matrix consisting of proteins and a high concentration of carbohydrates, especially hyaluronic acid. It is widely believed that hyaluronidase emanating from the sperm head plays a major role in penetration of the corona radiata, but the active swimming movements of the spermatozoa are also important. The zona pellucida, which is 13 µm thick in humans, consists principally of four glycoproteins—ZP1 to ZP4. ZP2 and ZP3 combine to form basic units that polymerize into long filaments. These filaments are periodically linked by cross-bridges of ZP1 and ZP4 molecules (Fig. 2.5). The zona pellucida of an unfertilized mouse egg is estimated to contain more than 1 billion copies of the ZP3 protein.
Transport of Gametes and Fertilization
Ovulation and Egg and Sperm Transport
Ovulation
Egg Transport
Sperm Transport
Formation and Function of the Corpus Luteum of Ovulation and Pregnancy
Fertilization
Penetration of the Corona Radiata
Attachment to and Penetration of the Zona Pellucida
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Transport of Gametes and Fertilization