Fig. 40.1
Seminoma in a needle biopsy of the retroperitoneum. a H&E stain. b OCT3/4 showing nuclear immunoreactivity. c CD117 showing cytoplasmic/membranous staining. d CD20 staining surrounding lymphocytes
Molecular Markers of Germ Cell Lineage
Through the years, a modest but significant amount of work has gone into understanding the molecular pathogenesis of testicular GCTs, the genomic differences among various tumor types, and the genes that are differentially expressed in chemotherapy-sensitive as well as chemotherapy-resistant tumors [1–3].
However, none of these findings are used clinically, either to establish a precise diagnosis (subclassification) or to choose therapy. At this time, the key to selecting proper therapy lies on an accurate pathological diagnosis, assessment of pathologic risk factors, state-of-the-art imaging, and serum tumor markers.
Testicular GCTs can be divided into three groups (infantile/prepubertal, adolescent/young adult, and spermatocytic seminoma (SS)), each with its own constellation of clinical histology, molecular, and clinical feature [4–7]. They originate from germ cells at different stages of development. Tumors arising in prepubertal gonads are either teratomas or yolk sac tumors (YSTs), tend to be diploid, and are not associated with i(12p) nor intratubular germ cell neoplasia unclassified (IGCNU). The annual incidence is approximately 0.12 per 100,000. SS arises in older patients. These benign tumors may be either diploid or aneuploid and have losses of chromosome 9 rather than i(12p). Intratubular SS is commonly encountered but IGCNU is not. Their annual incidence is approximately 0.2 per 100,000. The pathogenesis of prepubertal GCTs and SS is poorly understood.
The most common testicular GCTs arise in postpubertal men and are characterized genetically by the presence of excess genetic material of the short arm of chromosome 12, usually due to one or more copies of i(12p), or other forms of 12p amplification and aneuploidy [8] (Table 40.1). The consistent gain of genetic material from the short arm of chromosome 12 seen in these tumors suggests that it has a crucial role in their development. IGCNU is the precursor to these invasive tumors.
Table 40.1
Molecular characterization of testicular germ cell tumors (GCTs)
i(12p) is the most common genetic abnormality seen in testicular germ cell tumors |
|---|
Presence of i(12p) is characteristic but not entirely specific for germ cell tumor origin |
Presence of i(12p) is of diagnostic utility but does not predict histologic type, prognosis or response to therapy |
i(12p) is best identified by karyotype analysis |
Presence of i(12p) may be analyzed by fluorescent in situ hybridization (FISH) in formalin-fixed, paraffin-embedded tissues, but this assay suffers from less than ideal sensitivity and specificity |
While IGCNU is considered to be the precursor of all GCTs, the stage in germ cells development at which transformation occurs is not known. One model proposed by Skakkebaek and colleagues suggests that fetal gonocytes (primordial germ cells) undergo abnormal cell division (polyploidization) in utero, primarily due to environmental factors. These cells undergo abnormal cell division mediated by a kit receptor/kit ligand (stem cell factor) paracrine loop, leading to uncontrolled proliferation of gonocytes. Subsequent invasive growth may be mediated by postnatal and pubertal gonadotrophin stimulation. In this model, i(12p) is seen only after stromal invasion occurs [9]. A second model proposed by Chaganti and colleagues suggests that aberrant chromatid exchange events during meiotic crossing-over may lead to increased 12p copy number and overexpression of cyclin D2 ( CCND2). In a cell containing unrepaired DNA breaks (recombination associated), overexpressed cyclin D2 may block a p53-dependent apoptotic response and lead to reinitiation of cell cycle and genomic instability. This aberrant, genomically unstable cell is now able to escape the apoptotic effects of p53 and may re-enter the cell cycle as a neoplastic cell. In this model, i(12p) is present in IGCNU [10].
Given the fact that i(12p) or at least excess genetic material of 12p is characteristic of postpubertal GCTs of the testis, it should not come as a surprise that its presence can and has been used as a diagnostic assay. This genetic abnormality is not absolutely pathognomonic of germ cell neoplasia, yet it is a very useful diagnostic tool in selected circumstances due to its rare occurrence in other solid tumors [11–13]. Classically, i(12p) is a feature best seen by karyotype which by definition requires a metaphase spread (Fig. 40.2). However, in daily practice, this assay is rarely performed in solid tumors, given the popularity of modern molecular techniques. Because the possibility of germ cell origin is usually determined after the H&E slides are reviewed, many investigators have used fluorescent in situ hybridization (FISH) to establish the presence of i(12p) in a formalin-fixed, paraffin-embedded (FFPE) tumor-bearing tissue. In fact, multiple papers have been written documenting its utility [14–17] (Figs. 40.3 and 40.4). The most common assay is a library probe covering 12p, sometimes combined with a chromosome 12 centromeric probe. In some cases, a three-color probe is used that includes a portion of 12q. In theory, this approach is more scientifically sound since the goal is to identify excess genetic material of 12p rather than simply chromosomal gains commonly seen in any form of genomically unstable tumors. What all these studies have failed to do is to establish the sensitivity and specificity of their assay. As you can imagine, an assay that depends on the identification of abnormally large, irregular signals in a resting cell can be difficult to evaluate and is prone to problems in interpretation (Figs. 40.3 and 40.4). Now that other molecular assays that can utilize FFPE tissues have entered the diagnostic arena, it is imperative that we develop a more precise assay. One possibility that is being explored is using a single-nucleotide polymorphism (SNP) array, which has the advantage of providing very good copy number data as well chromosome gains and losses. While the price of this assay at the present time is high, the clinical penalty that is paid if this diagnosis is missed certainly merits the financial cost.
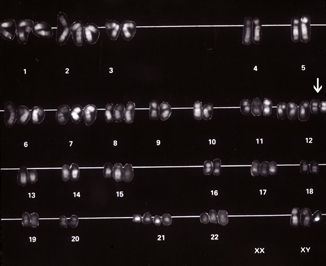
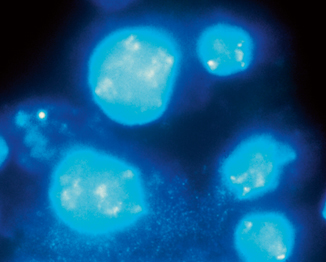
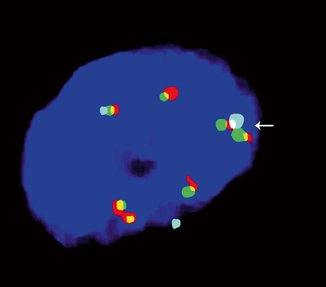

Fig. 40.2
An early karyotype depicting the presence of two copies of isochromosome 12p [i(12p)] ( arrow). It was these early studies that allowed investigators to define what we now regard as the most common genetic abnormality seen in adult onset testicular germ cell tumors (GCTs)

Fig. 40.3
Single-color FISH assay exhibiting large, irregular signals that may represent several copies of i(12p) ( left). Normal signal can be seen in an adjacent cell ( right)

Fig. 40.4
Three-color FISH probe exhibiting i(12p) ( arrow). The aqua centromeric probe is flanked by two red–green probes
Immunohistochemical Markers
Immunohistochemical markers can be a very powerful ancillary tool in classifying GSTs [18]. With experience, the overwhelming majority of tumors can be classified on high-quality H&E slides alone. However, the relative low incidence of these tumors is such that any given pathologist is likely to encounter no more than a handful of cases a year. This fact combined with the wide morphologic spectrum that can be seen in these tumors makes it very likely that the pathologist will be overly dependent on ancillary studies to arrive at a final diagnosis. It is best to first elaborate a differential diagnosis based on morphology and then decide what panel of antibodies should be ordered. Too often, I encounter cases in which virtually every marker associated with any type of GCT has been ordered, even though the differential diagnosis is rather limited. This “shotgun” approach should be avoided.
Intratubular Germ Cell Neoplasia (IGCNU)
IGCNU can be seen adjacent to invasive GCTs in virtually all cases in which residual testicular parenchyma is present [19]. It is present in up to 4 % of cryptorchid patients, up to 5 % of contralateral gonads in patients with unilateral GCT, and up to 1 % of patients biopsied for oligospermic infertility. Its association with testicular GCTs arising in prepubertal patients is still a source of controversy [4, 20, 21]. If present, it certainly does not have the same morphology or immunophenotype than what is seen in postpubertal gonads. Clinically, there are two settings in which it may be critical to confirm the presence of IGCNU: in the evaluation of a testicular biopsy at the time of infertility work up and when evaluating a testicular mass that is difficult to classify.
IGCNU cells contain glycogen and thus are PAS positive, diastase sensitive. Rarely will other intratubular cells, whether spermatogonia, spermatocytes, or Sertoli cells, show similar positivity. Placental-like alkaline phosphatase (PLAP) is one of the isoforms of alkaline phosphatase. PLAP antibodies will stain IGCNU, the majority of seminomas and ECs as well as a smaller percentage of YSTs. Immunoreactivity is seen in virtually all cases of IGCNU, and the staining pattern is usually membranous or cytoplasmic. No other non-neoplastic intratubular cells are immunoreactive for PLAP, but immunoreactivity may be seen in other types of nongerm cell malignancies [22, 23]. Because better markers have been developed over the years, we hardly ever use PLAP in our workup of testicular tumors. C-kit (CD117), a tyrosine kinase receptor expressed on stem cells, is overexpressed in a large percentage of IGCNU as well as seminomas, but not in other GCTs [24]. The staining pattern is cytoplasmic/membranous (Fig. 40.1c). Despite the overexpression of this antigen, CD117 is rarely mutated in these tumors. Care must be taken when interpreting CD117 in IGCNU since spermatogonia may occasionally express this antigen.
Other antibodies which immunoreact with IGCNU but are rarely used in clinical practice include M2A and 43-F [22, 25, 26]. POU5F1 (OCT3/4) is a very interesting marker with great clinical utility [27]. The gene serves as a transcription factor, and its product is expressed in pluripotent mouse and human embryonic stem cells and is down-regulated during differentiation. Since the gene is also required for self-renewal of embryonic stem cells, knocking out the gene is lethal. This antigen is expressed solely in IGCNU, seminoma, and EC, suggesting that these are the types of GCT cells with pluripotency, i.e., with capacity to differentiate. As a transcription factor, staining is localized to the nucleus (Fig. 40.1b).
Another transcription factor expressed in IGCNU is SALL4; however, this nuclear marker is expressed in a wider spectrum of GCTs including seminoma, EC, YST, and some glandular elements of teratoma [28]. As such, it is a useful marker in the characterization of GCTs but cannot be used in isolation. Podoplanin (clone D2-40), a transmembrane mucoprotein expressed on fetal germ cells, lymphatic endothelium, and mesotheliums, is an excellent cytoplasmic (membranous) marker with staining restricted to IGCNU and seminoma (Fig. 40.5) [29]. Since the expression patterns for CD117 and D2-40 overlap, there is no need to perform both assays.
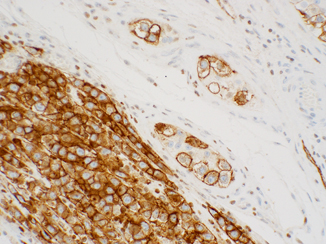

Fig. 40.5
Podoplanin (D2-40) showing strong cytoplasmic/membranous staining in intratubular germ cell neoplasia ( right) and seminoma ( left). This stain may be used interchangeably with CD117, but there is no need to perform both
There are several situations in which it is critically important to establish the presence or absence of IGCNU and in which immunohistochemistry may be of great utility. These include during the workup of infertility and in the presence of a poorly differentiated testicular neoplasm in order to establish germ cell lineage (Fig. 40.1a–d). However, there is absolutely no reason to perform tests for all of the markers mentioned above. While one antibody may be sufficient, I usually test for two antigens, OCT4 and either CD117 or D2-40. PAS, PLAP, M2A, and 43-F are of no additional clinical utility and I discourage their use. In this situation, SALL-4 adds no additional information to OCT4.
Seminoma
Seminomas are the most common GCTs arising in the male gonad, whether they arise in a pure state or mixed with other morphologic types [30–32]. “Pure” seminoma account for 30 % of testicular GCTs and another 15–20 % contain syncytiotrophoblasts without other germ cell components. Seminoma cells contain glycogen (PAS positive) and express PLAP, CD117, OCT3/4, and SALL-4 by immunohistochemistry but not cytokeratins, CD30, or inhibin (Table 40.2) [23, 33–37]. In fact, the immunophenotype of seminoma is virtually identical to IGCNU. In our practice, PAS and PLAP are rarely relied upon because of the availability of better discriminating markers. On occasion, weak CD30 cytoplasmic immunoreactivity may be encountered in isolated seminoma cells, a finding that should not warrant a change in diagnosis. It is important to keep in mind that CD30 may be expressed in some hematopoietic cells as well, so attention to nuclear detail is warranted. A minority of seminoma cells may express focal and weak, dot-like, or linear immunoreactivity for cytokeratin AE1/AE3 and CAM 5.2. However, there is never diffuse and strong staining throughout the cytoplasm. Caution must be taken when interpreting cytokeratin markers since syncytiotrophoblasts are usually strongly immunoreactive, as are their mononuclear variants. If one relies on panels of markers, this issue is resolved easily since syncytiotrophoblasts lack immunoreactivity to OCT4, SALL4, D2-40, etc. Like IGCNU, seminoma cells express OCT4 in a nuclear distribution [24, 37]. SALL4 is positive in a nuclear distribution, while CD117 and D2-40 are expressed in a cytoplasmic/membranous distribution, once again similar to ICGNU (Fig. 40.5).
Table 40.2
Immunohistochemical maker expression in primary testicular germ cell tumors (GCTs)
Marker | IGCNU | Seminoma | Embryonal Ca | Yolk sac tumor |
|---|---|---|---|---|
OCT4a | Positive | Positive | Positive | Negative |
CD117 | Positive, membranous | Positive, membranous | Negative (weak/focal positivity can be seen) | Negative (weak/focal positivity can be seen) |
D2-40a | Positive | Positive | Negative | Negative |
CD30a | Negative | Negative | Positive | Negative |
SALL4 | Positive | Positive | Positive | Positive |
Glypican 3a | Negative | Negative | Negative | Positive |
Cytokeratinb | Negative+ | Negative+ | Positive | Positive |
In practical terms, the panel of markers used to establish a diagnosis of seminoma will depend on the differential diagnosis. What is important to remember is that no other invasive GCTs will exhibit diffuse immunoreactivity for CD117 and D2-40, while seminoma shares OCT4 nuclear positivity with EC and SALL4 nuclear immunoreactivity with EC and YST.
Spermatocytic Seminoma
SSs are rare, comprising less than 2 % of testicular neoplasms [30]. They represent an entirely separate and distinct clinicopathologic entity from classic seminoma. The peak incidence is in the sixth decade of life (medial 54 years). Patients as old as 87 years and as young as 25 years of age have been affected. This tumor occurs only in the male gonad, is not associated with either cryptorchidism, other types of GCTs, or i(12p). Tumor cells do not contain glycogen (negative PAS stain). Immunohistochemical stains for PLAP are negative, although occasional cells may be weakly immunoreactive. Cytokeratin stains are negative, although occasional cells may exhibit dot-like cytoplasmic staining. CD30, OCT4, and podoplanin are negative, while some investigators have reported variable immunoreactivity for CD117 (C-kit) and SALL4 [24, 30, 38].
Embryonal Carcinoma
In its pure form, EC comprise up to 3 % of GCTs, although approximately 40 % of all GCTs contain an EC component. Over 50 % of tumors with either pure or predominant EC components will present with metastatic disease. Pure EC most commonly occurs in patients during the third or fourth decades of life, with an average age of 32 years and is extremely rare in prepubertal children [30]. Up to 10 % of patients present with symptoms related to metastatic disease. Metastases most commonly occur first to retroperitoneal lymph nodes. Twenty percent of patients present with metastatic disease with a smaller percentage also having supradiaphragmatic involvement.
The cells of EC usually show intense and diffuse immunoreactivity for PLAP, OCT4, SALL4, as well as cytokeratins (AE1/AE3 and Cam 5.2) (Fig. 40.6a–d). CD30 (Ber-H2) is a member of the tumor necrosis factor receptor family and has a cytoplasmic membrane and Golgi distribution. It is expressed exclusively in EC (Fig. 40.7). SOX-2 is a transcription factor involved in embryonic development and specifically plays a role in maintenance of pluripotency in undifferentiated embryonic stem cells [37, 39, 40]. It is expressed in a nuclear distribution in the majority of ECs but not seminoma (only isolated cases) or YSTs . While it may serve as an alternative to CD30, the assay is difficult to standardize in a CLIA complicate automated environment so we do not use it clinically. Only about 2 % of ECs react with epithelial membrane antigen (EMA). Staining for CD117 protein generally is negative, although some cells may exhibit low level of staining. β-HCG is demonstrable only in intermingled syncytiotrophoblastic cells [24]. Most AFP-positive foci in ECs probably represent unrecognized foci of YST or early transition to YST. Pertinent negatives include CD117 and D2-40, remembering that the former can rarely stain isolated cells .
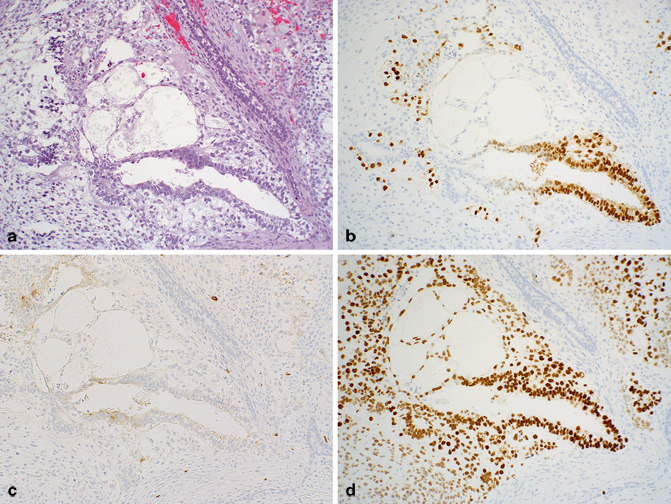
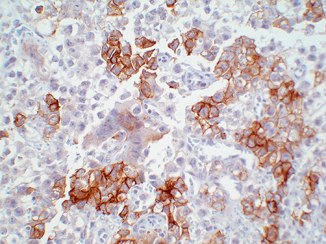

Fig. 40.6
Mixed germ cell tumor (GCT) composed of embryonal carcinoma (EC), yolk sac tumor (YST), and teratoma. a H&E stain. b OCT3/4 nuclear immunoreactivity is limited to EC. c CD117, although usually limited to seminoma, may be weakly positive in EC, as it is here. d SALL4 nuclear staining is seen in EC and YST but not in the epithelial teratomatous elements

Fig. 40.7
Cytoplasmic staining for CD30 in embryonal carcinoma (EC) while the adjacent seminoma cells are negative
Yolk Sac Tumor
YSTs are characterized by multiple patterns of growth that recapitulate the yolk sac, allantois, and extra embryonic mesenchyme. It has a bimodal age distribution: infants and young children and postpubertal males. In children, it commonly presents in its pure form, usually within the first 2 years of life. YSTs account for 75 % of childhood testicular GCTs. In the postpubertal setting, YST rarely presents in a pure form but is present in almost half of mixed GCTs [32, 41]. The incidence of a YST component is higher in primary mediastinal GCTs.
Tumor cells of YST are usually immunoreactive for low molecular weight cytokeratins and α-fetoprotein (AFP), a plasma protein produced by the yolk sac and liver in the fetus. We have found that AFP immunohistochemistry can be difficult to interpret. Staining can be weak, patchy, and it is often associated with a dirty background. While we admit that some of these findings could be laboratory associated, the fact of the matter is that we never perform or interpret this assay in isolation. Because we now have better antibodies, we do not consider this assay to be absolutely necessary. PLAP staining is variable and may be absent. As previously stated, we rarely perform this assay since it lacks discriminatory power. CD117, CD30, and OCT3/4 are usually negative, but SOX2, SALL4, and Glypican 3 are positive [24, 39, 42, 43]. Glypican 3 is a membrane-anchored heparin sulfate which expressed in virtually all YSTs and in the majority of choriocarcinomas (Fig. 40.8). Expression can be seen in the epithelial component of some teratomatous glands and some trophoblastic elements, but it is otherwise limited to YSTs.
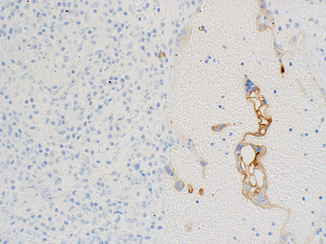

Fig. 40.8
Glypican 3 immunoreactivity in syncytiotrophoblasts adjacent to unreactive seminoma
Choriocarcinoma
Choriocarcinoma is composed of syncytiotrophoblastic, cytotrophoblastic, and other trophoblastic cells. It comprises less than 1 % of testicular GCTs in its pure form; however, it may be encountered as a component of a mixed GCT in up to 15 % of cases [30]. In its pure form, these highly malignant tumors occur in the second and third decades of life, are commonly associated with very high levels of serum HCG (usually above 50,000 mIu/mL), and exhibit metastatic disease at the time of initial presentation. In this setting, metastatic disease is found in not only the usual sites for other testicular tumors, but also via hematogenous spread to viscera, including lungs, liver, gastrointestinal tract, spleen, brain, and adrenal glands.
Syncytiotrophoblasts are immunoreactive with ß-hCG as well as inhibin, epithelial EMA, low molecular weight cytokeratins, and Glypican 3. Human chorionic gonadotrophic (hCG) is a dimeric glycoprotein that is produced by placental trophoblastic cells, predominantly the syncytiotrophoblasts. The beta-chain is unique to hCG and thus is the best antigenic marker for it. Essentially all choriocarcinomas are positive for β-hCG, but such reactivity is often limited to syncytiotrophoblasts or intermediate trophoblasts. Cytotrophoblasts are either negative or weakly positive for ß-HCG. PLAP may be positive but staining is variable [30]. Pregnancy-specific β1-glycoprotein and human placental lactogen also are positive in syncytiotrophoblasts and intermediate-sized trophoblasts but are negative in cytotrophoblasts [32, 44] .
Teratoma
The term teratoma refers to neoplasms composed of tissues, which have differentiated along any of the three somatic pathways: ectoderm, mesoderm, or endoderm [30, 32, 45]. Tumors composed of only one of these components are regarded as monodermal teratomas. Teratomas may be composed of mature tissues, embryonal-type tissues, or a mixture of both. Historically, they were subclassified as immature and mature forms based on their degree of differentiation. The World Health Organization (WHO) now recommends that these morphologies be considered as a single entity based on their overlapping genetic and clinical features [30] .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


