Modified-release oral drug delivery
Emma L. McConnell and Abdul W. Basit
Chapter contents
Modified-release oral drug delivery
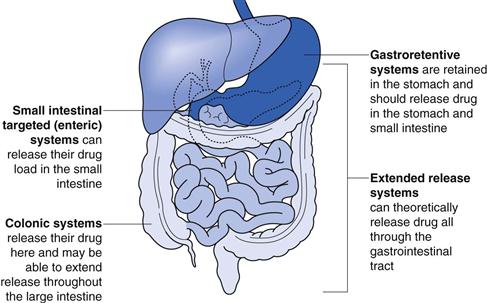
Sites of action for modified-release dosage forms and biopharmaceutical considerations
Designing a modified-release formulation: factors to consider
Key points
• Modified release can refer to extended, sustained, controlled, delayed or gastro-resistant release.
• Drugs can be targeted to the colon by using bacterial enzymes to initiate drug release.
Modified-release oral drug delivery
Administration of medicines by the oral route can seem like the most straightforward option for patients. It is the most commonly used route, with more than 70% of all medicines being delivered in this way. Oral medicines are easy to administer, improve patient compliance and are cheaper than some of the alternatives (e.g. injections). Most medicines administered by the oral route provide what is known as ‘immediate-release’ drug delivery or ‘conventional’ drug delivery. A common example would be the use of paracetamol (acetaminophen) for a headache; the tablet or capsule disintegrates quickly in the stomach fluids releasing the drug to provide rapid onset of effect, following absorption in the gastrointestinal tract. However, there are some situations in which this rapid onset is not desirable and a modification of the drug release pattern (or profile) is necessary to slow it down or make the drug’s effects last longer (e.g. for 24 hours). These more advanced oral drug delivery formulations are often referred to as oral modified-release (MR) drug delivery systems.
Modified-release drug delivery refers to the manipulation or modification of drug release from a dosage form (e.g. tablet, pellet, capsule) with the specific aim of delivering active pharmaceutical ingredients (API) at:
Modified-release drug delivery is a broad term which covers a variety of different approaches. These will be dealt with in further detail throughout this chapter. Briefly, the different types are:
The site of action of each of these systems is shown in Figure 31.1.
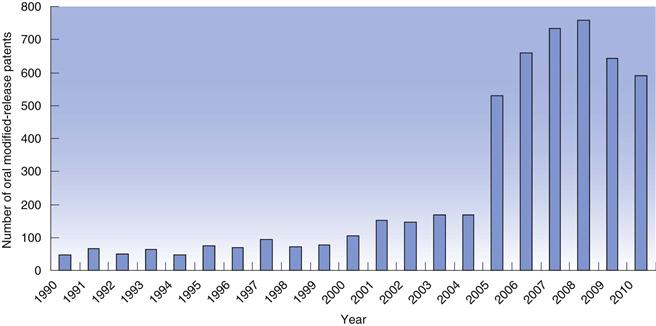
The concept of modified-release dosage forms has been around since the late 1800s when the idea of protecting the stomach from irritant drugs triggered a search for gastro-resistant materials. As knowledge of the gastrointestinal tract increased (pH, bacteria, transit times), the success and scope of the dosage forms targeted to the gastrointestinal tract improved. In recent years, there has been a huge increase in the number of patents filed for modified-release dosage forms (Fig. 31.2) highlighting the intense interest of the pharmaceutical industry in exploiting the benefits of these technologies to improve product performance.
What modified-release drug delivery means for the patient
Keeping drug in the therapeutic range.
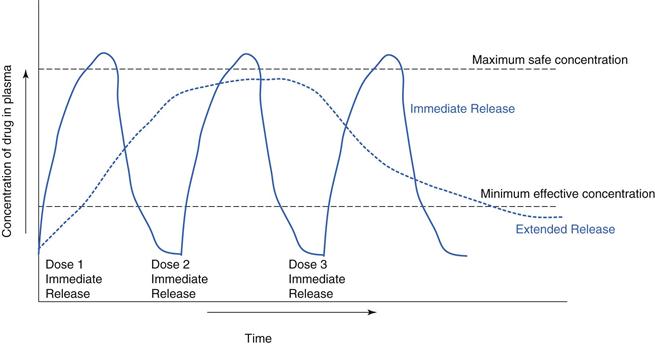
Modified release is often used to improve therapeutic outcomes for a patient relative to an immediate-release medication. For example, a drug which is rapidly absorbed and eliminated can have a steep plasma profile in an immediate-release formulation. An extended-release formulation can keep the drug at therapeutic levels for longer (Fig. 31.3). For many chronic illnesses, symptom breakthrough can occur if the blood concentration falls below the minimum effective concentration e.g. in asthma or depressive illness. This minimum level can also be critical for control of pain, consequently drugs, such as opioid analgesics are often given as extended-release preparations.
Maintaining drug levels overnight.
It is often not acceptable that patients be required to take medications during the night, with consequent loss of sleep. Overnight management of pain in terminally ill patients can be very important to maintain sleep.
Chronotherapy.
Timing the drug release to coincide with when it is required is known as chronotherapy. For example, a modified-release dosage form may be tailored to enable drug release to occur in the morning around the time of wakening, when symptoms of, for example, arthritis, asthma or allergies are often at their worst. A clinical study has shown that patients with arthritis had a better reduction in morning joint stiffness when they received modified-release prednisolone rather than a conventional dosage form.
Reducing side effects.
Immediate-release formulations can often have a high maximum concentration in the blood (Cmax). If Cmax is above the safety limit of the drug, adverse events may be more likely. Using modified-release formulations to reduce Cmax can reduce the incidence and severity of the side effects of some drugs. Additionally, some drugs, such as potassium chloride can be irritating to the gastrointestinal tract if delivered in an immediate-release bolus. A slow, sustained release is required to minimize the build-up of irritant concentrations.
Improving compliance.
A significant driver to developing a modified-release dosage form comes from trying to achieve once-daily dosing. Once-daily dosing is considered to be more convenient for patients and reduces the risk of missed doses throughout the day.
Treatment of local areas in the gastrointestinal tract.
Some conditions such as inflammatory bowel disease require topical treatment (e.g. with steroids) at the inflamed intestinal surface. Site-specific drug targeting (e.g. to the colon) can deliver the drug directly to its site of action.
What modified-release drug delivery means for the healthcare professional and pharmaceutical industry
Provides doctor, pharmacist and patient choice.
Healthcare professionals will be primarily concerned with the therapeutic advantages outlined above, but increasingly there is concern for personalized medicines and health services. A choice of immediate-release dosage forms and modified-release dosage forms can allow healthcare professionals to tailor treatment to their patients’ needs.
Product life extension.
Improving on current marketed formulations by employing modified-release technologies can sometimes enable pharmaceutical companies to extend a product’s patent life.
Higher development costs.
There are much higher costs for pharmaceutical companies in developing a modified-release formulation compared to a conventional immediate-release dosage form.
Cost savings to healthcare providers.
Cost-savings may be achieved from better disease management.
Sites of action for modified-release dosage forms and biopharmaceutical considerations
The gastrointestinal tract
Biopharmaceutical factors (i.e. the effect of the gastrointestinal physiology and environment on drugs and dosages forms) are considered in more detail in Chapter 19. Here some of the key biological factors that influence the in vivo behaviour of modified-release dosage forms are summarized and discussed. To understand these, the factors limiting drug bioavailability should be noted. The overall process of drug release and absorption will only be as fast as the slowest of many processes. The most common possible rate-limiting steps following oral administration of a solid dosage form are (1) drug release from the dosage form, (2) dissolution of the drug or (3) absorption of drug molecules.
pH
The stomach generally has a low pH and is therefore acidic. Gastro-resistant coated dosage forms are designed to be acid resistant. Some patients can have a higher stomach pH due to age, disease or ethnic origin which can affect dosage form disintegration and dissolution. This can result in premature drug release and/or dose dumping (dose dumping is the release of all the drug in one bolus).
Gastrointestinal pH generally increases in the small intestine, due to bicarbonate secretion. This is often used as a trigger for small intestinal drug delivery via gastro-resistant coating. The pH gradually increases to a maximum of about pH 7 at the ileo-caecal junction. In the colon, the pH drops slightly due to the production of short chain fatty acids by bacteria here, but gradually rises again distally. In some people, the pH does not get as high as pH 7 (and this may change from day to day). Therefore, if a polymer is used which dissolves at pH 7 (see below), then there is a good chance that the dosage form using this polymer will not dissolve, leaving a tablet intact and the patient without their dose. This has been observed in the clinic with some patients with ulcerative colitis.