Emulsions and creams
Gillian M. Eccleston
Chapter contents
Development of pharmaceutical emulsions
Emulsion theory related to pharmaceutical emulsions and creams
Selection of the emulsifying agent (emulsifier)
Emulsifying agents (emulsifiers)
Function of emulsifying agents
Classification of emulsifying agents
Natural macromolecular materials
Manufacture and processing of emulsions and creams
Identification of emulsion type
Stabilization by use of mixed emulsifiers
Evaluation of emulsion stability
Key points
Introduction
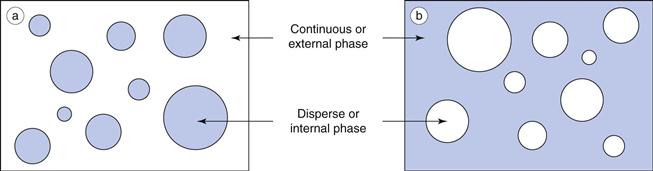
An emulsion is a dispersion of two immiscible (or partially miscible) liquids, one of which is distributed uniformly in the form of fine droplets (the dispersed phase) throughout the other (the continuous phase). The immiscible liquids are by convention described as ‘oil’ and ‘water’, as invariably one liquid is non-polar (e.g. an oil, wax or lipid) and the other is polar (e.g. water or aqueous solution). For simplicity and consistency, the terms ‘oil’ and ‘water’ are used in this context throughout this chapter.
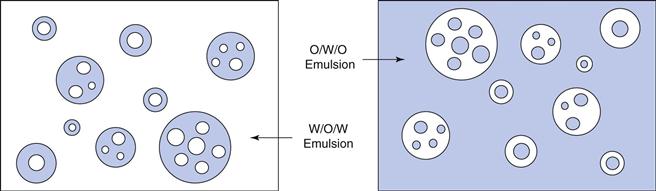
Oil-in-water emulsions (o/w) contain oil droplets dispersed in water, and water-in-oil emulsions (w/o) contain water droplets dispersed in oil (Fig. 27.1). Multiple emulsions can also be formed from oil and water by the re-emulsification of an existing emulsion to form two disperse phases. For example, multiple emulsions can be described as oil-in-water-in-oil (o/w/o). These are o/w emulsions which are further dispersed in an oil continuum. Conversely water-in-oil-in-water (w/o/w) type multiple emulsions can be prepared by further emulsification of a w/o emulsion in water (Fig. 27.2).
Emulsion formation
When two immiscible liquids are placed together in a container, they will form distinct layers with a minimum area of contact (interfacial area) between the two liquids. In this state, the surface free energy, G, is at a minimum. On mixing or mechanical agitation (i.e. input of energy) both liquids will form droplets of various sizes, thereby increasing the interfacial area between the liquids with a corresponding increase in the surface free energy of the system. Emulsions are therefore thermodynamically unstable.
The increase in surface free energy ΔG, brought about by the formation of droplets and the corresponding increase in surface area ΔA is given in Eqn 27.1 in which γ is the surface (or interfacial) tension.
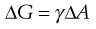 (27.1)
(27.1)
In order to reduce this surface free energy, the droplets assume a spherical shape; this gives a minimum surface area per unit volume. On contact droplets will coalesce (merge and re-combine) in an attempt to reduce the total interfacial area (and thus total surface energy, as indicated by Eqn 27.1).
Thus, emulsification can be considered to be the result of two competing processes that occur simultaneously. The first process requires energy input to disrupt the bulk liquids and form fine droplets thereby increasing the free energy of the system. The second process, which involves the coalescence of droplets, occurs spontaneously to reduce the interfacial area and minimize the free energy. If agitation ceases altogether, coalescence will continue until complete phase separation is obtained, the state of minimum free energy.
Droplet diameters vary enormously in pharmaceutical emulsions, but typically cover the range 0.1 µm (100 nm) to 25 µm. The visual appearance of an emulsion reflects the influence of droplet size on light scattering, and varies from transparent or translucent for emulsions composed of small nano-sized droplets (less than approximately 200 nm) to milky white and opaque for emulsions containing larger droplets.
Since emulsions are thermodynamically unstable, they will revert back to separate oil and water continuous phases unless kinetically stabilized by the addition of emulsifying agents (see sections below on ‘Emulsifying agents (emulsifiers)’ and ‘Emulsion stability’).
Partially miscible liquids
It should be noted, that when oil and water phases are partially miscible, droplet growth with eventual phase separation may occur by Ostwald ripening (see later) rather than coalescence. Ostwald ripening is an irreversible process which involves the growth of large droplets at the expense of smaller ones; it is considered later in this chapter in the section on ‘Emulsion stability’. Ostwald ripening does not require any contact between droplets and is an important mechanism of instability in sub-micrometre pharmaceutical emulsions.
Emulsions in pharmacy
Emulsions can be formulated for virtually all the major routes of administration, although most commercial products are developed for the oral, parenteral and topical routes. Oral and intravenous emulsions are almost exclusively of the o/w type, whereas dermatological emulsions, and emulsions for subcutaneous or intramuscular injection may also be formulated as w/o emulsions.
Medicinal o/w emulsions for oral administration have a long tradition of use to deliver medicinal oils for the local treatment of constipation (e.g. mineral oil, castor oil) and as oral food supplements (e.g. fish liver oils and vegetable oils) in a more palatable and acceptable form. The unpleasant taste of the oil is masked by the aqueous phase and any odour is suppressed when it is administered as the internal phase of an o/w emulsion.
Oil-in-water emulsions containing vegetable oils are also used for the oral delivery of drugs and vitamins of low aqueous solubility. Intestinal absorption is generally enhanced when an oily solution of drug is presented in the form of small sub-micrometre oil droplets, because of the larger interfacial area available for contact at the absorption site. Absorption is also generally faster and more complete than from suspension or tablet forms, because the drug in oral emulsions is already solubilized in the oil, thus eliminating the dissolution step prior to absorption.
Oral drug delivery using emulsions can be unpredictable because emulsions may become unstable in the low pH environment of the stomach. Emulsion concentrates, described as self-emulsifying drug delivery systems (SEDDS), are available commercially to minimize instability. SEDDS are composed of drug, oil(s), surfactants and sometimes co-solvents. They are not themselves emulsions, but form an emulsion on mild agitation in the aqueous environment of the stomach.
Sterile intravenous lipid o/w emulsions are used clinically as a source of calories and essential fatty acids for debilitated patients. Such emulsions (e.g Intralipid®) are also used as intravenous drug carriers for drugs of limited water solubility; marketed products are available for drugs such as diazepam (Diamuls®), propofol (Diprovan®), vitamin K (Phytonadione®) and docetaxol (Aventrix®). The advantages of such intravenous emulsions over solution formulations (in which the drug is solubilized by various co-solvents, and/or surfactants and/or pH control) include, a higher drug payload, lower toxicity, less pain on injection and protection of labile drugs by the oily environment.
Emulsions incorporating contrast agents (iodized oils, bromized perfluorocarbon oils) are used in diagnostic imaging including X-ray examinations of body organs, computed tomography and magnetic resonance imaging.
Water-in-oil emulsions administered by the subcutaneous or intramuscular routes can be used to prolong the delivery of water-soluble antigens and thus provide a longer lasting immunity. The antigen or drug must first diffuse from the aqueous droplets through the oily external phase before it reaches the tissues. Such emulsions are sometimes difficult to inject because of the high viscosity of the oily continuous phase. Multiple w/o/w emulsions, which are less viscous, have also been investigated for the prolonged release of drugs and vaccines incorporated in the innermost aqueous phase (see Chapter 36).
Dermatological emulsions are the largest class of emulsions used in pharmacy, and range in consistency from structured fluids (lotions, liniments) to semisolids (creams). Both oil-in-water and water-in-oil emulsions are extensively used as vehicles to deliver drugs to the skin, and for their therapeutic properties. Patient acceptance of such formulations is based on sensory attributes such as appearance, texture and ‘skin feel’. Water-in-oil emulsions tend to be greasy, and although this conveys a greater feeling of richness, w/o emulsions do not mix well with aqueous wound exudates and are also sometimes difficult to wash off the skin. They do however hydrate the skin by occlusion, an important factor in drug permeation. In contrast o/w lotions and creams readily mix with tissue exudates and are more easily removed by washing.
Dermatological emulsions (Chapter 39) facilitate drug permeation into and through the skin by occlusion, by the incorporation of penetration enhancing components and/or by evaporation on the skin surface. As most o/w creams are applied and rubbed onto the skin as a thin film, the drug delivery system is not the bulk emulsion, but rather a dynamic evaporating film in which the dissolution and partitioning environment alters as the relative concentrations of the volatile ingredients change. Rapid evaporation may temporarily supersaturate the film increasing thermodynamic activity and drug permeation.
Whilst dermatological emulsions and creams are two-phase systems, single-phase systems, including ointments and gels, are also available for topical application. These are described in Chapter 39.
Development of pharmaceutical emulsions
Although emulsions have many distinct advantages over other dosage forms, often improving bioavailability and reducing side effects, there are relatively few commercial oral or parenteral emulsions available. This comparative lack of usage is due to the fundamental problems of maintaining emulsion stability. Unstable emulsions are unsightly, give unpredictable drug-release profiles and may be toxic, for example droplet size increases in parenteral emulsions may cause thrombosis following injection. However, there is currently a large increase in research into all aspects of emulsions, although as yet there are few new products. This resurgence of interest, which is mainly focused on lipid emulsions for local or intravenous delivery, combines nano-science with the drive for cell-selective drug targeting and delivery.
Nanoemulsions
Nomenclature relating to nanoemulsions
It is necessary to spend a little time here considering the nomenclature of nanoemulsions as unfortunately there is some confusion in the literature and definitions may change.
Conventional emulsions (macroemulsions) and nanoemulsions.
According to the convention for nanoscale materials, nanoemulsions are defined in the wider literature as clear or transluscent emulsions containing droplet sizes typically below ~200 nm (0.2 µm). In the pharmaceutical literature however, confusion arises because the term nanoemulsion is sometimes used to include milky white emulsions containing droplets of up to 500 nm (0.5 µm) in diameter. In this chapter, milky white emulsions containing sub-microscopical droplets of less than a micrometre will be called colloidal, submicron (sub-micrometre) or ultrafine emulsions, whilst the term nanoemulsion will be reserved here for transparent emulsions containing droplet diameters less than ~200 nm.
Microemulsions and nanoemulsions.
The interchangeable use of the terms microemulsion and nanoemulsion is a more serious error that is becoming increasingly common in the pharmaceutical literature, causing confusion and inaccurate reporting. Although both microemulsions and nanoemulsions are clear and transparent, they are structurally quite different. Nanoemulsions are thermodynamically unstable dispersions of oil and water that contain individual small droplets. In contrast, so-called microemulsions are not emulsions. They are thermodynamically stable, single-phase systems that form spontaneously and have a number of different microstructures depending on the nature and concentration of the components (see also Chapter 5).
Properties of nanoemulsions
Nanoemulsions are relatively stable physically, as the droplets do not collide as frequently as in ordinary emulsions and their small droplet sizes enable them to penetrate deep into the tissues through fine capillaries. Thus, such emulsions are being investigated extensively as drug carriers and for their ability to target specific sites in the body including the liver and the brain. The surface properties of emulsions can be modified by controlling the charged nature of the interfacial film or by incorporating homing devices into the film to target specific tissues and organs after injection.
Negatively charged droplets are cleared more rapidly from the blood than neutral or positively charged ones. Lipid emulsions modified with apo-E specifically target the paranchymal cells of the liver and cationic emulsions complexed with plasmid DNA show promise in gene delivery.
Positively charged (cationic) nanoemulsions have also been shown to improve skin permeation of poorly soluble antifungal drugs and ceramides due to their interaction with the negatively charged skin epithelia cells. Water-in-oil nanoemulsion formulations are under investigation in cancer chemotherapy for prolonging drug release after intramuscular or intratumoral injection, and as a means of enhancing the transport of anticancer agents via the lymphatic system.
Emulsion theory related to pharmaceutical emulsions and creams
The classical theories of emulsification for simple two-phase oil and water model emulsions based on droplet interactions and interfacial films are considered in Chapter 5. However commercial pharmaceutical emulsions (even dilute mobile fluids for intravenous administration) are rarely such simple oil and water systems. They are more often complex multiphase emulsions containing additional phases (e.g. liquid crystalline) to oil and water. A unified theory of emulsification cannot be applied quantitatively to such multiphase emulsions, which range in consistency from mobile or structured fluids to soft or stiff semisolids.
Formulation of emulsions
When formulating a pharmaceutical emulsion, the choice of oil, emulsifier and emulsion type (o/w, w/o or multiple emulsion) will depend on the route of administration and its ultimate clinical use. The formulator must optimize processing conditions as these control droplet size distributions and rheology, which in turn influence emulsion stability and therapeutic response. The potential toxicity of all the excipients, their cost and possible chemical incompatibilities in the final formulation must also be identified. It is sometimes difficult to isolate these effects in practical emulsions as each is dependent on, and influenced by, the other. Thus ingredient selection is made often by trial and error and is dependent on the experience of the formulator.
Selection of the oil phase
The oil used in the preparation of pharmaceutical emulsions may be the medicament itself or it may function as a carrier for a lipid-soluble drug. The selection of the oil phase will depend on many factors including the desired physical properties of the emulsion, the miscibility of the oil and aqueous phases, the solubility of the drug (if present) in the oil and the desired consistency of the final emulsion. Some oils, in particular unsaturated oils of vegetable origin, are liable to auto-oxidation and become rancid, and so antioxidants or preservatives must be incorporated into the emulsion to inhibit this degradation process.
For externally applied emulsions, oils based on hydrocarbons are widely used. Liquid paraffin, either alone or combined with soft or hard paraffin, is used in numerous dermatological lotions and creams, both as a vehicle for the drug, and for the occlusive and sensory characteristics imparted when the emulsion is spread onto the skin. Turpentine oil, benzyl benzoate and various silicone oils are examples of other externally applied oils that are formulated as emulsions.
In oral emulsions, the most widely used medicinal oils are castor oil and liquid paraffin, which are non-biodegradable and provide a local laxative effect in the gastrointestinal tract, fish liver oils (e.g. cod or halibut) that are high in vitamins A and D or various fixed oils of vegetable origin (e.g. arachis oil) as nutritional supplements. Vegetable oils are also used as drug carriers as they are readily absorbed in the gastrointestinal tract. The oil phase is rarely inert, as it may influence bioavailability by its influence on gastric emptying time.
The choice of oil is severely limited in emulsions for parenteral administration, as many are inherently toxic. Although purified mineral oil is used in some water-in-oil depot preparations for intramuscular injection, where its potential toxicity (e.g. abscess formation at the injection site) is balanced against efficacy, it is too toxic to be incorporated into intravenous emulsions. A range of purified vegetable oils have been used, almost exclusively over many years in lipid emulsions for parenteral nutrition and as intravenous carriers for drugs of limited aqueous solubility.
The purified vegetable oils used in parenteral products comprise mixtures of long-chain triglycerides (LCTs) containing C12-C18 saturated and unsaturated fatty acid moieties, mainly oleic, linoleic, palmitic and stearic acids. Although a large number of vegetable oils have been investigated as possible stable, non-toxic oils for use in lipid emulsions, most commercial products contain soya bean or safflower oils because of their high content of the essential fatty acid, linoleic acid. Medium chain triglycerides (MCTs) which contain shorter fatty acid moieties (~C6-C10) are obtained by the re-esterification of fractionated coconut oil fatty acids (mainly capric and caprylic) with glycerol. These provide a more rapidly available source of energy, as well as enhancing the solubilizing capacity for lipid soluble drugs, including ciclosporin.
Mixtures containing both long and medium chain triglycerides have been adopted in some commercial preparations (see Table 27.1). Structured triglycerides, formed by modifying the oil enzymatically to produce 1,3-specific triglycerides with a mixture of long chain and medium chain fatty acids within the same molecule are under investigation as possible alternatives to physical mixtures of LCTs and MCTs.
Table 27.1
Selected commercial lipid emulsions for parenteral nutrition
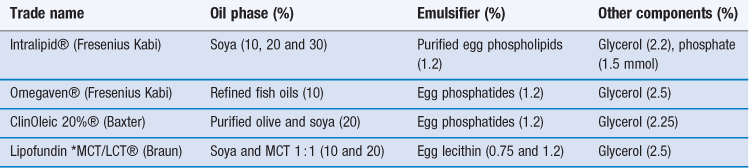
*MCT = medium chain triglyceride; LCT = long chain triglyceride
Emulsified perfluorochemicals are also considered acceptable for intravenous use provided that they are excreted relatively quickly. A major problem in the formulation of the early perfluorocarbon emulsions as blood substitutes was that the oils that formed the most stable emulsions were not cleared rapidly from the body.
Selection of the emulsifying agent (emulsifier)
Emulsifiers are used to control emulsion stability during a shelf-life that can vary from days for extemporaneously prepared emulsions, to months or years for commercial preparations. In practice, combinations of emulsifiers rather than single agents are generally used. The choice of emulsifier depends on the type of emulsion to be prepared, emulsifier toxicity (or irritancy if applied to the skin) and potential cost and availability. The final clinical use of the emulsion is also an important consideration, as emulsifiers control the in-vivo fate of emulsions by their influence on droplet size distribution, and the charge and surface properties of the individual droplets.
The functionality and types of emulsifying agent are of such importance to the properties of the emulsion that emulsifiers are considered in a separate major section below headed ‘Emulsifying agents (emulsifiers)’.
Other excipients
Preservatives
The aqueous continuous phase of an oil-in-water emulsion can produce ideal conditions for the growth of bacteria, moulds and fungi. The potential sources of contamination may be from the water used, the raw materials (especially if these are natural products), the manufacturing and packaging equipment, or introduced by the patient during use. Such contamination, which may constitute a health hazard, can also affect the physicochemical properties of the formulation, causing colour, odour or pH changes and even phase separation. Water-in-oil emulsions are less susceptible to such contamination because the aqueous phase is essentially enclosed and protected by the oil.
An ideal preservative should exhibit a wide spectrum of activity against bacteria, yeasts and moulds; it should be free from toxic, irritant or sensitizing activity (Chapter 50). Large-volume injectable fat emulsions do not contain preservatives and sterilization is achieved by autoclaving without a preservative. Phenoxyethanol, benzoic acid, and the parabenzoates are used as preservatives in oral and topical emulsions. The preservative will partition between the oil and aqueous phases, with the oil phase acting as a reservoir. Aqueous pH is an additional factor to be considered, as a sufficient concentration of the unionized form must be present to ensure proper preservation. Compatibility problems can occur between emulsifiers and preservatives, for example polyoxyethylene non-ionic surfactants emulsifiers and phenolic preservatives, not only destroying their microbial activity but also the emulsification properties of the surfactant.
Antioxidants and humectants
Antioxidants are added to some emulsions to prevent oxidative deterioration of the oil, emulsifier or the drug itself during storage. Such deterioration imparts an unpleasant, rancid odour and taste. Some oils are supplied containing suitable antioxidants. Antioxidants commonly used in pharmacy include butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) at concentrations up to 0.2%, and the alkyl gallates, which are effective at very low concentrations (0.001-0.1%). Alpha-tocopherol is added to some commercial lipid emulsions to prevent peroxidation of unsaturated fatty acids.
Humectants, such as propylene glycol, glycerol and sorbitol at concentrations up to 5%, are often added to dermatological preparations to reduce the evaporation of the water from the emulsion during storage and use. However, high concentrations may also remove moisture from the skin, causing dryness.
Emulsifying agents (emulsifiers)
Function of emulsifying agents
The function of an emulsifying agent (emulsifier) is to maintain the dispersion state of the emulsion for an extended period of time after the cessation of agitation, i.e. to impart kinetic stability to the emulsion. The dispersed droplets do not retain their initial character because the emulsion becomes thermodynamically stable (for the free energy is still high) but rather because the added emulsifiers inhibit or delay the processes of coalescence and Ostwald ripening (described below).
Emulsifiers generally impart time-dependent stability by the formation of a mechanical or electrostatic barrier at the droplet interface (an interfacial film) or in the external phase (a rheological barrier). The formation of interfacial films by adsorption of the emulsifier at the oil/water interface has been discussed in Chapter 5.
The interfacial film may increase droplet-droplet repulsion by the introduction of electrostatic or steric repulsive forces to counteract the van der Waals forces of attraction. Electrostatic repulsions are important in o/w emulsions stabilized by ionic emulsifiers, whereas steric repulsive forces, which arise when hydrated polymer chains approach one another, dominate with non-ionic emulsifiers and in w/o emulsions. The interfacial film may also provide a mechanical barrier to prevent droplet coalescence, particularly if it is close packed and elastic. Generally, mixtures of emulsifiers provide stronger interfacial films. Surfactant emulsifiers lower the interfacial tension between the oil and water. Although this facilitates the formation of droplets during emulsification and reduces the thermodynamic tendency for coalescence, interfacial tension reduction is not a major factor in maintaining long-term stability.
Interfacial films do not have the dominant role in maintaining stability in many practical emulsions in which the external phase is thickened by the emulsifier, i.e. in which the emulsifier significantly increases the viscosity of the continuous phase. In these, the structured continuous phase forms a rheological barrier which prevents the movement and hence the close approach of droplets. Emulsifiers that thicken the external phase but do not form an interfacial film are variously described as auxiliary emulsifiers, co-emulsifiers or viscosity enhancers. Many pharmaceutically important mixed emulsifiers, including lecithin and the emulsifying waxes, form interfacial films at low concentration and also structure the external phase at higher concentrations by the formation of additional lamellar liquid crystalline (with lecithins) or crystalline gel network phases (with emulsifying waxes).
Emulsion type
The type of emulsion that forms (whether o/w or w/o or multiple emulsion) and droplet size distribution depend on a number of interrelated factors, including the method of preparation (energy input), the relative volumes of the oil and water phases and the chemical nature of the emulsifying agent. When oil and water are mixed vigorously in the absence of an emulsifier, droplets of both liquids are produced initially, with the more rapidly coalescing droplets forming the continuous phase. Generally this is the liquid present in the greater amount because the greater number of droplets formed increases the probability of droplet collision and subsequent coalescence. With the inclusion of an emulsifier, the type of emulsion that forms is no longer a function of phase volume alone, but also depends on the relative solubility of the emulsifier in the oil and water phases. In general, the phase in which the emulsifying agent is more soluble (or in the case of solids, more easily wetted by) will form the continuous phase. Thus, hydrophilic surfactants and polymers promote o/w emulsions and lipophilic emulsifiers (with low HLB, see ‘Emulsifier selection’ section below) promote w/o systems.
Theoretically, the disperse phase of an emulsion can occupy up to a maximum of 74% of the phase volume. Whilst such high internal phase o/w emulsions stabilized by suitable emulsifiers have been produced, it is more difficult to form w/o emulsions with greater than 50% disperse phase because of the steric mechanisms involved in their stabilization. In practice, pharmaceutical emulsions usually contain 10-30% disperse phase.
Classification of emulsifying agents
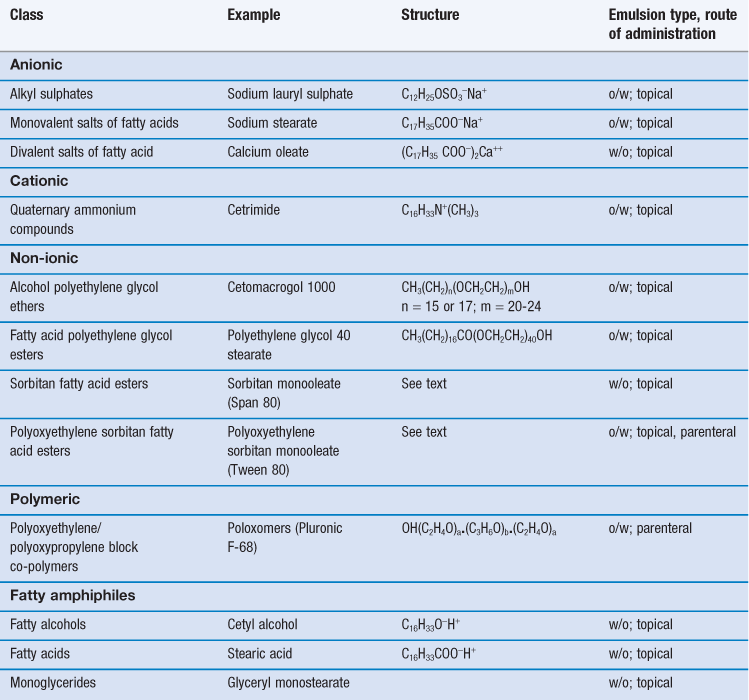
Emulsifying agents may be classified into two groups (i) synthetic or semi-synthetic surface active agents and polymers and (ii) naturally occurring materials and their derivatives. Examples of typical pharmaceutical emulsifying agents are shown in Tables 27.2 and 27.3.
Table 27.3
| Class | Example | Emulsion type; route of administration |
| Polysaccharide | Acacia | o/w; oral |
| Methylcellulose | o/w; oral | |
| Phospholipid | Purified lecithins | o/w; oral, parenteral |
| Sterol | Wool fat | w/o; topical |
| Cholesterol and its esters | w/o; topical | |
| Finely divided solid | Bentonite | o/w and w/o; topical |
| Aluminium hydroxide | o/w, oral |
Surface active agents and polymers
Surface active agents (surfactants for short) are further classified as ionic (i.e. anionic or cationic) or non-ionic according to their characteristics on dissociation. Most are mixtures of long chain homologues having hydrocarbon chain lengths between 12-18 carbon atoms with a hydrophilic head group. Their emulsifying power is influenced by batch variations in the homologue composition, with pure homologue surfactants proving to be very poor emulsifiers.
There are an enormous number of synthetic surfactants available commercially, and they form by far the largest group of emulsifiers studied in the general scientific literature. Unfortunately, the majority of the synthetic surfactants are toxic (many are haemolytic) and irritant to the skin and the mucous membranes of the gastrointestinal tract. In general, cationic surfactants are the most toxic and irritant and non-ionic surfactants the least. Thus, for pharmaceutical emulsions, ionic synthetic surfactants are used only in external topical preparations where they are present at relatively low concentration. Both ionic and non-ionic surfactants are combined with fatty alcohols to produce anionic, cationic or non-ionic emulsifying waxes, which are used to both stabilize and structure aqueous lotions and creams. A limited number of non-ionic surfactants (e.g. the polysorbates, discussed below) are also used internally in oral and parenteral emulsions, although lecithin (a mixture of anionic and neutral phospholipids) is the main emulsifier in commercial lipid emulsions. The non-ionic block co-polymer poloxomer 188 (Pluronic® F68) has been used in perfluorochemical emulsions for intravenous infusion, although some patients are sensitive to this emulsifier.
Anionic surfactants
Anionic surfactants dissociate at high pH to form a long-chain anion with surface activity. Emulsifying properties are lost and emulsions are unstable in acid conditions and in the presence of cationic materials, such as cationic surfactants and polymers. Examples of anionic surfactants include:
Alkyl sulphates.
Sodium lauryl (dodecyl) sulphate is the most widely used surfactant in topical products. The commercial sulphate is actually a mixture containing predominantly the C12 homologue, but also contains some C14 and C16 homologues. Sodium lauryl sulphate alone is a weak emulsifier of the o/w type, but forms a powerful o/w blend when it is used in conjunction with cetostearyl alcohol.
Monovalent salts of fatty acids.
Emulsifiers in this group consist mainly of the alkali salts of long chain fatty acids, e.g. C17H35COO−X+, where X may be Na, K, NH4 or triethanolamine (TEA). Alone, these ‘soaps’ promote rather unstable, mobile o/w emulsions, but when combined with fatty acids they form powerful o/w emulsifying blends that stabilize a number of dermatological products.
In many formulations, the ‘nascent soap’ method of preparation is used, in which soap is formed in situ from the partial neutralization of a fatty acid (which may be a component of the oil phase) with the appropriate alkali. For example, in white liniment, ammonium oleate is formed in situ from the reaction between ammonia solution and oleic acid. Triethanolamine soaps, formed in situ by the partial neutralization of fatty acid (generally stearic acid) by TEA have a long history of use in the formulation of cosmetic and pharmaceutical o/w vanishing creams.
Divalent salts of fatty acids.
Calcium salts of fatty acids containing two hydrocarbon chains form w/o emulsions due to their limited solubility in water. These are generally formed in situ by the interaction of calcium hydroxide with a fatty acid. In zinc cream, calcium oleate is formed in situ from the interaction between oleic acid and calcium hydroxide. This approach is also used in some formulations of oily calamine cream, in which oleic acid and some of the free fatty acid component of arachis oil are partially neutralized by calcium hydroxide to form a calcium oleate/oleic acid mixed emulsifier.
Cationic surfactants
Cationic surfactants dissociate at low pH to form a long-chain surface-active cation. Emulsions containing cationic surfactant as emulsifier are unstable at high pH and in the presence of anionic materials including anionic surfactants and polymers.
Quaternary ammonium compounds.
These constitute an important group of cationic emulsifiers in dermatological preparations because they also have antimicrobial properties. Cetrimide (cetyltrimethyl ammonium bromide) is blended with cetostearyl alcohol to form cationic emulsifying wax which is the mixed emulsifier used in cetrimide cream.
Non-ionic surfactants
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree