CHAPTER 44 Mitosis and Cytokinesi
Mitosis is the division of a somatic cell (a vegetative cell in yeast) into two daughter cells. The daughters are usually identical copies of the parent cell, but the process can be asymmetrical. For example, division of stem cells gives rise to one stem cell and another daughter cell that goes on to mature into a differentiated cell. See Box 41-1 for examples.
Traditionally, mitotic events are subdivided into six phases: prophase, prometaphase, metaphase, anaphase, telophase, and cytokinesis (Fig. 44-1). The dramatic reorganization of both the nucleus and cytoplasm during the mitotic phases is brought about by activation of a number of protein kinases, including Cdk1–cyclin B–p9 (abbreviated here as “Cdk1 kinase”; see Chapter 40). After activation by Cdc25 phosphatase, Cdk1 kinase accumulates in the nucleus, where it joins Cdk1–cyclin A, which was activated somewhat earlier (see Chapter 43). These two Cdk1 kinase complexes operate both as master controllers and as workhorses that directly phosphorylate many proteins whose functional and structural status is altered during mitosis.
Mitosis is an ancient eukaryotic process, and a number of variations emerged during evolution. Many single-celled eukaryotes, including yeast and slime molds, undergo a closed mitosis, in which spindle formation and chromosome segregation occur within an intact nuclear envelope to which the spindle poles are anchored. This chapter focuses on open mitosis, as used by most plants and animals, in which the nuclear envelope disassembles before the chromosomes segregate. Figure 44-2 summarizes some of the important events during the various mitotic phases.
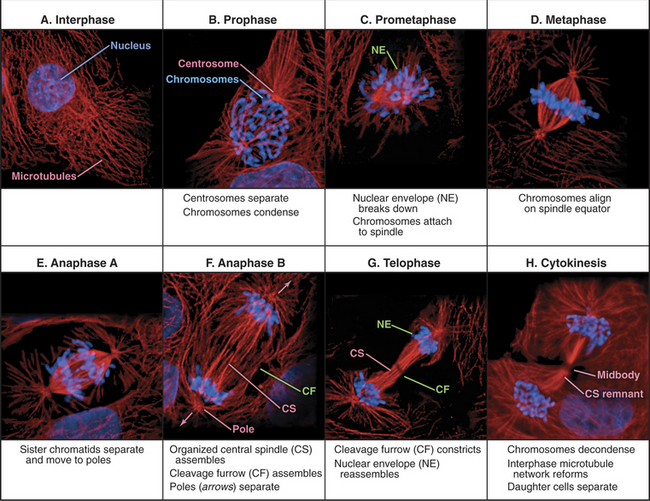
Figure 44-2 overview of the phases of mitosis and definition of the most important terms. a–c, Prophase–prometaphase: The Cdk1 kinases trigger condensation of replicated sister chromatids, disassembly of the nuclear envelope and Golgi, and a dramatic reorganization of the cytoskeleton. These changes abolish the barrier between the chromosomes and cytoplasm. As cytoplasmic microtubules contact the condensed chromosomes, they attach at the kinetochores (see Fig. 13-20). Interaction of motor proteins on the chromosomes with microtubules produces jostling movements that culminate with the chromosomes aligned at the midplane of a bipolar scaffolding of microtubules (the spindle). D–F, Metaphase–anaphase: Once all of the chromosomes achieve a bipolar attachment to the spindle, an inhibitory signal is switched off. This leads to activation of a proteolytic network that destroys proteins responsible for holding sister chromatids together and also inactivates Cdk1 by destroying its cyclin B cofactor (see Fig. 40-18). These changes trigger separation of the sister chromatids, which then move toward opposite spindle poles. G–H, Telophase–cytokinesis: Targeting of nuclear envelope components back to the surface of the chromatids subsequently leads to the re-formation of two daughter nuclei. In most cells, the two daughter nuclei and the surrounding cytoplasm are partitioned by cytokinesis following the contraction of an actin-myosin ring.
(Micrographs courtesy of William C. Earnshaw.)
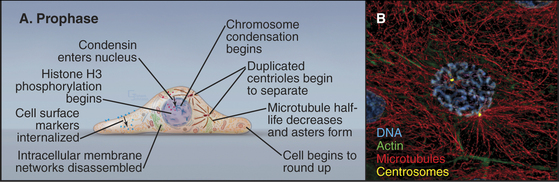
Prophase
Prophase, the transition from G2 into mitosis, begins with the first visible condensation of the chromosomes and disassembly of the nucleolus (Fig. 44-3). In the cytoplasm, the interphase network of long microtubules centered on a single centrosome (see Fig. 34-17) is converted into two radial arrays of short microtubules called asters. Most types of intermediate filaments disassemble, the Golgi and endoplasmic reticulum fragment, and both endocytosis and exocytosis are curtailed.
Nuclear Changes in Prophase
Chromosome condensation, the landmark event at the onset of prophase, often begins in isolated patches of chromatin at the nuclear periphery. Later, chromosomes condense into two threads, termed sister chromatids, which are closely paired along their entire lengths. Although chromosome condensation was first observed more than a century ago, the biochemical mechanism remains a mystery. Protein kinases are thought to drive mitotic chromosome condensation by phosphorylating a number of the hundreds of proteins associated with mitotic chromosomes. The onset of condensation correlates with phosphorylation of histones H1 by Cdk1 kinase and H3 by Aurora-B protein kinase, and both are widely used as physiological markers for mitotic cells. However, chromosome condensation still occurs when both of these phosphorylation events are blocked. Possibly, some combination of histone modifications might provide a “code” that promotes chromatin condensation (see Fig. 13-3).
Two pentameric protein complexes, condensin I and II, are major constituents of mitotic chromosomes with an essential role in chromosome architecture (see Fig. 13-19). The two complexes share a pair of ABC ATPases, SMC2, and SMC4 (structural maintenance of chromosomes) but have two different sets of three auxiliary proteins. Condensin II enters the cell nucleus during prophase, where it is required for prophase chromosome condensation. However, chromosomes of vertebrate cells that lack condensin condense rapidly once the nuclear envelope breaks down at the onset of prometaphase, so condensin is not directly responsible for mitotic chromosome condensation. Chromosomes that lack condensin separate normally at the beginning of anaphase but appear to fall apart while moving toward the spindle poles. This appears to reflect defects in their underlying structure, and condensin is required for proteins of the chromosome scaffold to assemble properly (see Fig. 13-19). The molecular explanation for these effects is not known. In vitro, condensin complexes can promote coiling and compaction of DNA, but the significance of this is also unknown.
Cytoplasmic Changes in Prophase
Most of the cytoskeleton reorganizes during prophase. Most notably, the microtubule array changes from an extensive network permeating the cytoplasm into two dense, radial arrays of short, dynamic microtubules around the duplicated centrosomes (see Chapter 34). Each of these asters eventually becomes one pole of the mitotic spindle. During prophase, the two asters usually migrate apart across the surface of the nucle-ar envelope, signaling the start of spindle assembly (Fig. 44-3).
Mitotic microtubules behave like interphase microtubules in many ways (see Chapter 34). They are nucleated at their minus ends, they grow by addition of tubulin subunits at their free plus ends, and they undergo random catastrophes during which they rapidly shorten. To a large extent, the prophase changes in microtubule organization can be explained by two simple biochemical changes: (1) increased microtubule-nucleating activity of centrosomes and (2) altered dynamic instability properties of the microtubules (Table 44-1; also see Chapter 34). Interphase microtubules have a high probability of recovering from catastrophes, so they grow quite long. Mitotic microtubules grow more rapidly but exist only transiently. This is because when they undergo a catastrophe, they usually shorten all the way back to the centrosome, with little chance of rescue. These differences in dynamic instability can be reproduced in vitro in mitotic and interphase cellular extracts. They appear to arise, at least in part, from counterbalancing interactions between microtubule-associated proteins, which promote microtubule stability, and kinesin-13 (see Fig. 36-13), which promotes microtubule disas-sembly.
Table 44-1 COMPARISON OF MICROTUBULE DYNAMICS IN INTERPHASE AND MITOTIC NEWT LUNG CELLS
| Parameter | Interphase | Mitosis |
|---|---|---|
| Elongation rate | 7 μm/min | 14 μm/min |
| Elongation time before catastrophe | 71 s | 60 s |
| Shortening rate | 17 μm/min | 17 μm/min |
| Probability of rescue from catastrophe* | 0.046/s | 0 |
| Length | 100 μm | 14 μm |
* Most cellular microtubules grow constantly by addition of subunits to their free ends but occasionally stop growing and begin shrinking rapidly (a “catastrophe”). Unless shrinking is reversed (a “rescue”), the microtubule completely disappears.
(Data from Gliksman NR, Skib-bens RV, Salmon ED: How the transition frequencies of microtubule dynamic instability regulate microtubule dynamics in interphase and mitosis. Mol Biol Cell 4:1035–1050, 1993.)
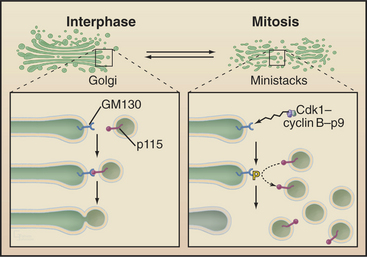
The Golgi apparatus and endoplasmic reticulum fragment or vesiculate during prophase (Fig. 44-4). In ad-dition, many membrane-mediated events, including fluid-phase pinocytosis, endocytosis, exocytosis, and intracellular sorting of membrane components (see Chapters 21 and 22), greatly decrease. Golgi disassembly is driven by several kinases, including Cdk1. The first step of the process is fragmentation of the Golgi into smaller mini-stacks. The second step is still being investigated. Some evidence argues that Cdk1 phosphorylation of key components prevents the fusion of transport vesicles back into Golgi stacks (see Chapter 21), the net result being that the Golgi buds apart into small vesicles. Other evidence suggests that an imbalance of vesicle flow between the Golgi and the endoplasmic reticulum results in the Golgi being absorbed into the ER during mitosis. Whatever the mechanism of its disassembly, Golgi reassembly begins again during anaphase, following inactivation of Cdk1 kinase. Some Golgi derived vesicles contribute to the plasma membrane during cleavage furrow ingression at the end of mitosis (see later).
Prometaphase
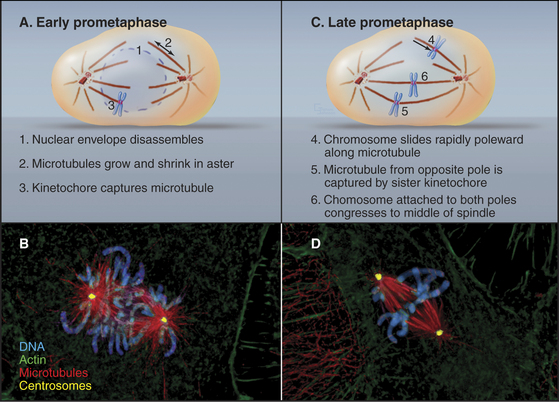
In cells that undergo an open mitosis, prometaphase begins abruptly with disassembly of the nuclear envelope (Fig. 44-5). Microtubules growing outward from the spindle poles penetrate holes in the nuclear envelope, make contact with the chromosomes, and attach to them at specialized structures called kinetochores (see Fig. 13-20). Interactions of the two opposing kinetochores of paired sister chromatids with microtubules from opposite poles of the spindle ultimately result in alignment of the chromosomes in a group midway between the poles. An important cell cycle checkpoint (see Chapter 40) known as the spindle checkpoint delays the onset of chromosome segregation until any attachment errors have been corrected and all chromosomes have achieved a bipolar attachment.
Nuclear Envelope Disassembly in Prometaphase
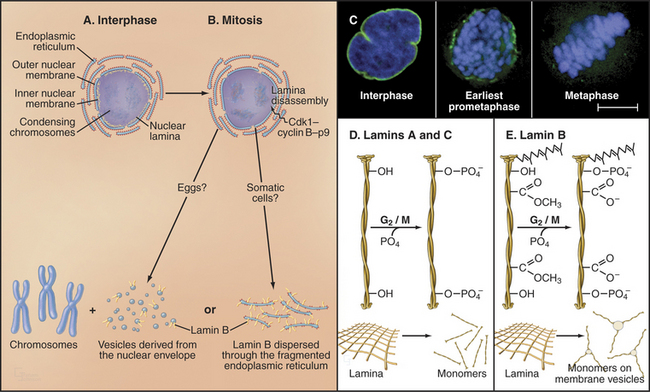
Nuclear envelope disassembly involves the removal of two membrane bilayers coupled with disassembly of the nuclear pores and the fibrous nuclear lamina meshwork that underlies the inner bilayer (Fig. 44-6). Protein phosphorylation triggers breakdown of the nuclear envelope but the critical targets and mechanisms are not entirely known. Phosphorylation of the nuclear lamins at two sites flanking the central coiled-coil causes the lamina network to disassemble into subunits and might contribute to disassembly of the envelope. Cdk1 kinase can phosphorylate these lamin residues in vitro, but other kinases might participate in vivo. Additionally, phosphorylation of nucleoporins leads to nuclear pore disassembly. This fenestrates the nuclear envelope, dissolving the barrier between nucleus and cytoplasm. Interaction between microtubules and dynein associated with the nuclear envelope may also rip holes in the envelope.
Nuclear envelope components are dispersed in the cytoplasm from prometaphase until telophase (Fig. 44-6), but the mechanism may differ in various cell types. In fertilized amphibian eggs, the nuclear membrane breaks up into small vesicles that disperse in the cytoplasm. In vertebrate somatic cells, the nuclear envelope may be absorbed into the endoplasmic reticulum, which remains as an extensive tubular network throughout mitosis. In both cases, lamin B remains associated with the dispersed nuclear envelope, whereas lamins A and C and many proteins of the nuclear pore complexes disperse as soluble subunits.
During prophase, kinetochores transform from nondescript balls of condensed chromatin into organized plaques on the surface of the chromosomes. By early prometaphase, the characteristic trilaminar disk structure (see Fig. 13-20) can be seen. Each sister chromatid has a kinetochore. Thus, sister kinetochores are located on opposite faces of the mitotic chromosome. Only one of the two sister kinetochores faces a given spindle pole at any one time.
Organization of the Mitotic Spindle
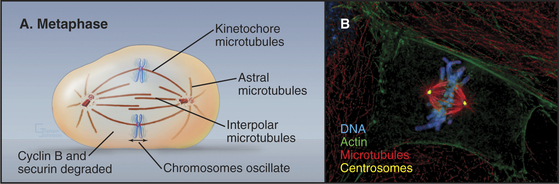
The mature metaphase spindle is a bilaterally symmetrical structure with centrally located chromosomes flanked by arrays of microtubules radiating from the poles (Fig. 44-7).
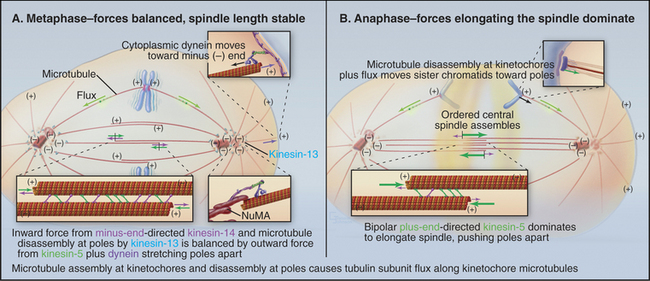
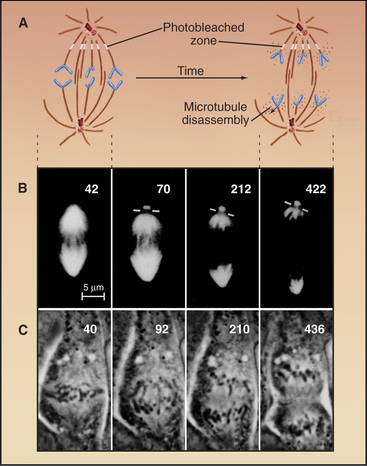
Three predominant classes of microtubules are present in the metaphase spindle (Fig. 44-12). Kinetochore microtubules have their plus ends embedded in the kinetochore and their minus ends at or near the spindle pole. They characteristically form bundles, called kinetochore fibers, which contain anywhere from 1 microtubule in the budding yeast to more than 200 microtubules in some higher plants. Each human kinetochore binds about 20 microtubules. Up to about 80% of the approximately 2200 spindle microtubules in humans may be present in kinetochore fibers. Interpolar microtubules are distributed throughout the body of the spindle and do not attach to kinetochores. Their minus ends may terminate near the pole but are not physically linked to it so that they appear to be free at both ends. Many interpolar microtubules penetrate between and through the chromosomes and extend for some distance beyond them. Thus, the central spindle contains a large number of interdigitated antiparallel microtubules. Tracking these spindle microtubules by electron microscopy has revealed a tendency for the interdigitated microtubules of opposite polarity to pack next to one another. During late anaphase, these antiparallel microtubules bundle to form a structure, called the central spindle, that appears to have important roles during cytokinesis. Astral microtubules project out from the poles and have a role in orienting the spindle in the cell through interactions with the cell cortex. All of the microtubules within each aster have the same polarity, with their minus ends proximal to the pole. Each unit of a spindle pole, with its associated kinetochore and interpolar and astral microtubules, is referred to as a half-spindle.
Spindle structure is largely determined by a combination of microtubule dynamics plus the action of at least seven different types of kinesins plus cytoplasmic dynein (see Chapter 36). These motors often work in opposition to one another. As a result, the spindle is a highly dynamic structure whose morphology changes as the balance of forces shifts between the various motors. For example, inactivating one or more kinesins with drugs or switching a temperature-sensitive mutant to the nonpermissive temperature can cause the spindle to collapse rapidly on itself. Consequently, chromosomal movements and changes in spindle morphology are complex processes that reflect both the dynamic growth and shrinkage of microtubules plus the net vectorial output of multiple antagonistic and synergistic motors. These various components interact; for example, force exerted by motors can influence the dynamic assembly/disassembly of microtubules.
Spindle Assembly
In metazoans, spindle assembly starts in prophase with the separation of the asters. In most cells, each aster is organized around a centrosome, consisting of a centriole pair and associated pericentriolar material. γ-Tubulin ring complexes in the pericentriolar material efficiently nucleate microtubules (see Fig. 34-16), so each aster acts as a microtubule organizing center. By the end of prophase, the spindle consists of two asters linked by a few interpolar microtubules. Cytoplasmic dynein at the cell cortex exerts an outward force separating the asters, whereas kinesin-14 motors (which move toward microtubule minus ends) on the interpolar microtubules exert a counterbalancing force holding the asters together.
This balance of forces changes when the nuclear envelope breaks down. Bipolar kinesin-5 motors are phosphorylated by Cdk1 kinase and concentrate in the central spindle, where they cross-link adjacent antiparallel interpolar microtubules. Kinesin-5 moves toward the plus ends of microtubules. If such a motor attaches to two adjacent antiparallel microtubules and begins to move, it will cause them to slide apart (Fig. 44-7). Thus, the action of kinesin-5 motors pushes the spindle poles apart. The two half-spindles do not separate because they are physically linked via the chromosomes, with sister kinetochores attached to opposite spindle poles.
Also at this time, the asters mature into focused spindle poles. The pericentriolar material efficiently nucleates the assembly of new microtubules with their minus ends at the pole. In addition, cytoplasmic dynein transports free microtubules that are nucleated throughout the cytoplasm to the centrosome along astral microtubules for incorporation into the spindle. The focused microtubule array at the pole forms partly owing to the tethering of microtubules by centrosomes, and partly due to the concerted action of various motors and microtubule cross-linking proteins such as nuclear mitotic apparatus protein (NuMA). NuMA is released from the nucleus on nuclear envelope breakdown, and it accumulates near the poles at the minus ends of microtubules.
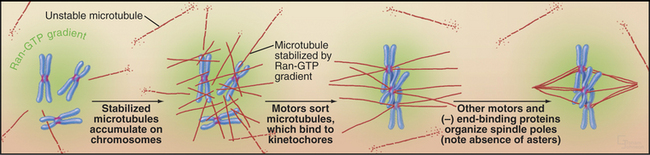
In large cells that lack centrosomes, such as eggs, spindle formation depends on an alternative pathway that is also active in cells with centrosomes (Fig. 44-8). Chromosomes stabilize nearby microtubules, which are then organized into a bipolar spindle by motor proteins and NuMA. This spindle assembly pathway involves importin a and b, two proteins that direct traffic through nuclear pores during interphase (see Fig. 16-14). Importin a and b inhibit mitotic spindle formation by sequestering several essential proteins, including NuMA. Chromosomes counteract this by releasing spindle assembly factors such as NuMA from importin a and b. The mechanism depends on the association of the GTP exchange factor RCC1 (Ran-GEF in Fig. 44-17) with chromosomes. RCC1 produces a local gradient of the active GTPase Ran-GTP, which dissociates NuMA from importin a and b just as it does during protein import into the nucleus. In this case, however, the net result is microtubule stabilization and assembly of the mitotic spindle.
Chromosome Attachment to the Spindle
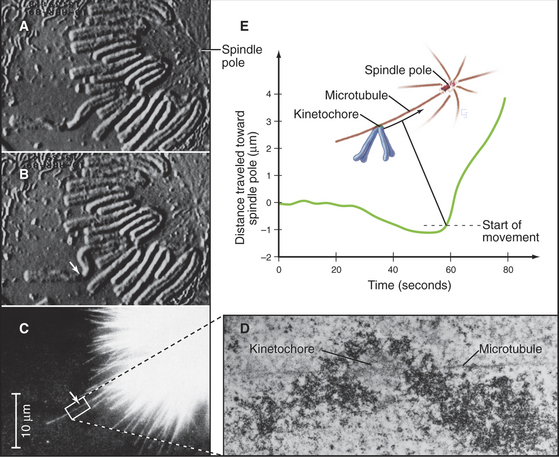
Dynamic microtubules of prometaphase asters scan the cytoplasm searching for both binding sites that will capture and stabilize their distal plus ends and for other components, including free microtubules. Breakdown of the nuclear envelope makes the condensed chromosomes accessible to the microtubules. Chance encounters with kinetochores during cycles of growth result in the plus ends of microtubules being captured by the kinetochore. Capture probably involves the nine-component KMN complex, which contains two components capable of binding weakly to microtubules. One of these, the helical Ndc80 complex (see Fig. 13-21) binds along the sides of microtubules forming fine hairs visible in the electron microscope. Other members of the complex provide an anchoring site for Ncd80 in the kinetochore. Captured microtubules are about five-fold less likely to depolymerize catastrophically than free microtubules. When catastrophes do occur, the microtubules depolymerize back to the pole, recycling tubu-lin subunits for incorporation into other, growing microtubules.
Initial attachment of a chromosome to a microtubule often involves a lateral interaction between the corona region of the kinetochore (see Fig. 13-20) and the side of a microtubule (Fig. 44-9). Cytoplasmic dynein then slides the chromosome rapidly along the microtubule toward the pole. These steps were first seen in animal cells, and a similar pattern of chromosome attachment and movement also occurs in budding yeast cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree