CHAPTER 14 Microscopic Anatomy of the Zygapophysial Joints, Intervertebral Discs, and Other Major Tissues of the Back
Much of the current anatomic research related to the spine is concerned with the zygapophysial (Z) joints and the intervertebral discs (IVDs). The gross anatomy of these structures is covered in detail in Chapter 2. The characteristics of these structures unique to the cervical, thoracic, and lumbar regions are covered in Chapters 5, 6, and 7, respectively. Because much of the current investigation related to the Z joints and IVDs has been carried out in the lumbar region, Chapter 7 describes the Z joints and IVDs in significant detail. However, a considerable amount of the research on these two tissues is associated with their microscopic anatomy and molecular structure. The results of these investigations provide a greater understanding of normal, as well as pathologic, structure and function at the microscopic, ultrastructural (electron microscopic), and molecular levels.
The purpose of this chapter is to provide the reader with comprehensive information on the microscopic anatomy of the Z joints and IVDs. A discussion of the normal composition of connective tissue in general and, more specifically, of hyaline cartilage and fibrocartilage in association with the Z joints and IVDs is also included. Finishing out the chapter is a brief overview of the microscopic anatomy of the other major tissues in the region of the back.
MICROSCOPIC ANATOMY OF THE ZYGAPOPHYSIAL JOINTS
As with all diarthrodial joints, the articular surfaces that form the Z joints are covered with shiny hyaline cartilage. This cartilage is lubricated by synovial fluid that allows the bones to glide smoothly over each other with minimal friction (Swann et al., 1974). A tough sleeve of dense connective tissue envelops the articular cartilages and joint cavity of the Z joints posteriorly. This connective tissue sleeve is known as the fibrous capsule. Anteriorly the ligamentum flavum takes the place of the articular capsule of the Z joint (Xu et al., 1991). A thin inner layer of highly vascularized connective tissue called the synovial membrane lines the joint capsule. Cells within the synovial membrane manufacture the synovial fluid.
This section discusses the microscopic anatomy of the articular cartilage, capsule, and synovial membrane of the Z joints. A working knowledge of connective tissue is important in treating pain of spinal origin because most tissues involved in the formation of the Z joints (and IVDs) are connective tissue, and pain arising from the Z joints is a significant cause of back pain (Mooney and Robertson, 1976; Kirkaldy-Willis, 1988). Therefore a section on connective tissue, including hyaline cartilage, immediately follows this section on Z joints.
Zygapophysial Joint Articular Cartilage
General Considerations.
The articular cartilages lining the superior and inferior articular processes of each Z joint are similar in many respects to the articular cartilage associated with most synovial joints of the body. This means that the articular cartilage lining each of the articular processes of a Z joint is made up of a special variety of hyaline cartilage that is durable, lubricated by synovial fluid, compressible, and also able to withstand large compressive forces (Williams et al., 1995).
The purposes of Z joint articular cartilage is to protect the articular surfaces of the superior and inferior articular processes by acting as a shock absorber and to allow the articular surfaces to move across one another with little friction. Both functions are carried out efficiently. In fact, the coefficient of friction for typical articular surfaces is less than 0.002, which means that the two surfaces of a typical Z joint glide across each other with much greater ease than they would if they were both made of ice (the coefficient of friction for ice sliding on ice is <0.03) (Triano, 1992).
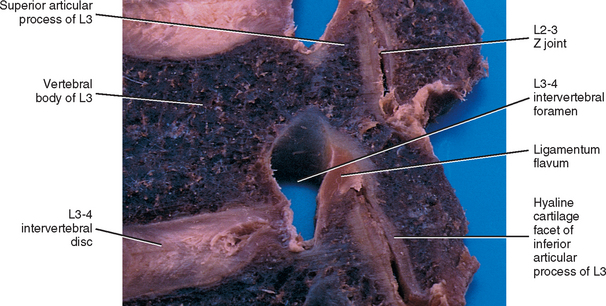
The articular cartilage of a single Z joint surface is small; in fact, the lumbar articular surfaces measure approximately 8 × 10 mm (Giles, 1992a,b). The Z joint articular cartilage also is approximately 1 to 2 mm thick (Figs. 14-1, 14-2, and 14-3). The concavity of the cartilage on lumbar superior articular facets is thicker than the periphery of the same surfaces. This is the opposite from that typically found in other joints of the body where the concavity of a joint surface usually is lined by thinner cartilage than that surrounding the concavity.
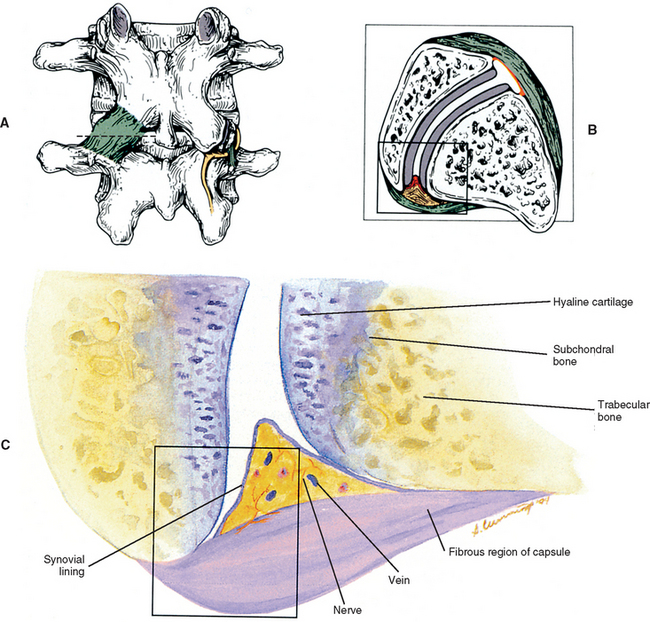
FIG. 14-1 Zygapophysial (Z) joint. A and B, Z joint from a posterior view and a horizontal section, respectively. C, Z joint after magnification by roughly a factor of 10. The articular cartilage, subchondral bone, and articular capsule are prominently displayed. In addition, a Z joint synovial fold is prominent. Notice that the articular capsule has an outer, tough fibrous region. The center of the synovial fold is more vascular and contains adipose tissue. A nerve can be seen passing through this latter region. A synovial lining can be seen on the deep surface of the articular capsule and the synovial fold. The box enclosing much of the synovial fold and a portion of the superior articular process is the region shown at higher magnification in Figure. 14-7.
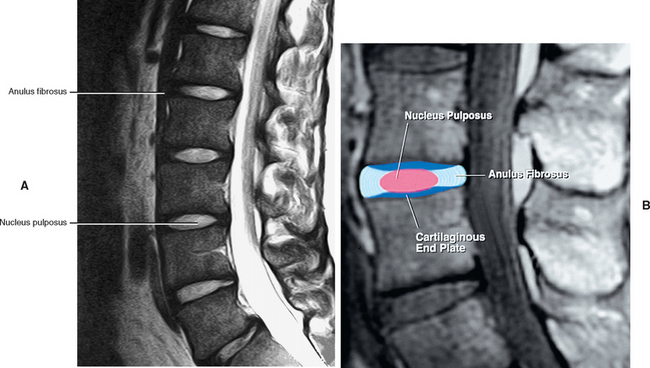
FIG. 14-3 Magnetic resonance imaging scan of the lumbar region performed in a parasagittal plane. The plane of section roughly corresponds to that of Figure. 14-2. Notice the intervertebral discs, the intervertebral foramina, and their contents.
(Magnetic resonance image courtesy Dr. Dennis Skogsbergh.)
Z joint articular cartilage is made up of 75% water and 25% solids (Giles, 1992a,b) and consists of cells embedded in an abundant and firm matrix (Fig. 14-4). The cells that produce the cartilage matrix are chondroblasts, and in mature cartilage they are known as chondrocytes (Table 14-1). The matrix is made up of an intricate network of collagen fibers surrounded by proteoglycans and glycoproteins. The concentration of these constituents of articular cartilage differs from one part of the joint surface to another and also at different depths from the joint surface (Giles, 1992a,b).
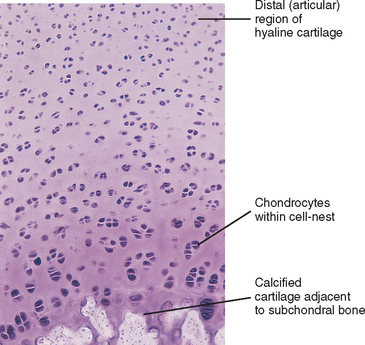
Fresh hyaline cartilage is bluish white and translucent. In stained, fixed preparations, the matrix appears glassy, homogeneous, and smooth. Distributed throughout the matrix are spaces called lacunae, and within each lacuna is a chondrocyte. As with all articular cartilage, that of the Z joints has no nerve supply and no direct blood supply. Chondrocytes must receive nutrients by diffusion across the cartilage matrix from several sources. These sources include the blood vessels within the synovial membrane that is located along the peripheral margin of the nonarticular portion of the cartilage, the synovial fluid, and the blood vessels in the adjacent bone (Williams et al., 1995).
Chondrocytes are found singly in the lacunae. Commonly, especially in cartilage that is actively growing, the lacunae are grouped into clusters of two or more. These clusters are called cell nests or isogenous cell groups (Fig. 14-5). The cells within the nests have arisen from the mitotic activity of a single chondrocyte; therefore the presence of isogenous cell groups signifies interstitial cartilage growth. This is supported by electron microscopy findings revealing that the chondrocytes within a cell nest exhibit a well-developed rough endoplasmic reticulum, a Golgi complex, and a large amount of glycogen and lipid.
Articular cartilage differs from typical hyaline cartilage in that the articular surface does not possess a covering of perichondrium (Giles, 1992b; Williams et al., 1995). Instead the cells of the articular surface appear flat and are closer together than they are farther within the cartilage matrix. In addition, the matrix of the articular surface becomes dense and fibrous. The collagen fibers, which course perpendicular to the articulating surface from deep within the cartilage matrix, curve as they reach the joint surface and become oriented parallel to the free edge of the articular cartilage.
Cartilage Matrix.
Collagen.
Collagen functions to bind the cartilage together, protect the chondrocytes, allow for attachment of the articular cartilage to the subchondral bone, and help resist compressive loads (Giles, 1992a). Because collagen is an important constituent of all the connective tissue components of the Z joints and the IVDs, it is discussed in detail in Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components.
Ground substance.
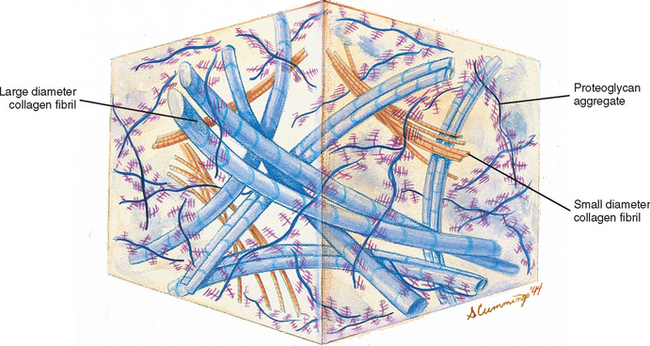
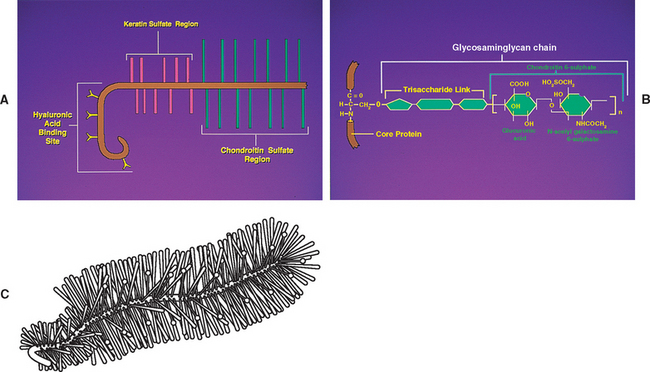
Chemical analysis of the ground substance of the extracellular matrix of hyaline cartilage reveals that it contains a small amount of glycoproteins and a high concentration of three types of glycosaminoglycans: hyaluronic acid, chondroitin sulfate, and keratan sulfate. The chondroitin and keratan sulfates are joined to a core protein to form a proteoglycan monomer. These macromolecules interact with the collagen and elastic fibers of the hyaline cartilage matrix (Fig. 14-6).
The single core protein of the proteoglycan molecule has a molecular weight of 200, 000 to 350, 000 Da. The core proteins represent approximately 7% to 12% of the dry weight of cartilage. Bound to each core protein are 80 to 100 chondroitin 4-sulfate and chondroitin 6-sulfate chains, each with a molecular weight of 20, 000 Da. These two glycosaminoglycans make up 80% to 85% of the dry weight of hyaline cartilage. In addition, approximately 50 chains of keratan sulfate, each with a molecular weight of 5000 Da, are also attached to the core protein. Keratan sulfate contributes approximately 7% of the total dry weight of hyaline cartilage.
At one end of each core protein is a hyaluronic acid-binding region (see Fig. 14-6). At this site the proteoglycan units are joined to hyaluronic acid molecules to form long proteoglycan-hyaluronic acid (PG-HA) aggregates. The interaction of the proteoglycan monomer with hyaluronic acid is strengthened by the presence of a link protein (see Fig. 14-6). Proteoglycans and glycosaminoglycans are discussed in further detail later in this chapter with regard to the IVD.
Chondronectin is a glycoprotein found in cartilage. Glycoproteins differ from proteoglycans by their low carbohydrate content, different repeating disaccharide units, and the absence of sulfate esters. Chondronectin participates in the adhesion of chondrocytes to type II collagen. Common glycoproteins found in other body tissues include laminin and fibronectin. Laminin is found in basal laminae and is partially responsible for the adhesion of epithelial cells. Fibronectin is found in blood, plasma, fibroblasts, and some epithelial cells and helps to mediate normal cell adhesion and migration (Table 14-2).
Clinical and biomechanical considerations.
Normally fluid moves out of articular cartilage when it is compressed and back into the cartilage when the Z joint is distracted. Such movement may help nutrients diffuse through the matrix to the chondrocytes. Articular cartilage can deform considerably when heavy compressive loads are applied to a joint. However, it returns to its previous state when the load is removed. If injured, articular cartilage heals rather slowly (a 1-mm defect heals in approximately 4 weeks). Passive movement of the joint may stimulate cartilage regeneration, whereas immobility results in the development of adhesions. Intermittent light weight-bearing activity does not stimulate cartilage regeneration but does stop the development of adhesions (Triano, 1992).
Articular cartilage becomes yellow, thinner, and more brittle with age, and undulations that may develop into ragged projections appear as a result of “wear and tear” of the joint surface (Williams et al., 1995). Also with age, fissures or cracks may develop in the articular cartilage. The development of such fissures is known as fibrillation of articular cartilage. The fissures may extend from the joint surface to the subchondral bone.
Zygapophysial Joint Articular Capsule
The Z joint capsules attach to the margins of the opposed superior and inferior articular facets of adjacent vertebrae throughout the vertebral column. The capsules are longer and looser in the cervical region than in the lumbar and thoracic regions. The articular capsule of a typical Z joint covers the joint’s posterolateral surface. It consists of an outer layer of white and shining dense fibroelastic connective tissue with bundles of collagen fibers coursing parallel with one another. Deep to the outer fibrous layer is a vascular central layer that is softer and more extensible than the outer layer, and is made up of elastic fibers, similar to the ligamentum flavum, areolar tissue, and loose connective tissue. The third and deepest layer of the Z joint capsule is an inner smooth and shining layer consisting of a white synovial membrane (Giles and Taylor, 1987; Yamashita et al., 1996). The outer, connective tissue layer of the capsule is tough and is essentially made up of parallel bundles of collagen fibers that are primarily oriented in the horizontal plane. A few fibroblasts and fibrocytes and a small amount of ground substance also are found in this layer (see Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components). The collagen fibers of the capsule attach to the adjacent surfaces of the superior and inferior articular processes, just peripheral to the articular cartilage. In fact, a gradual transition occurs from the joint capsule to fibrocartilage and finally to the articular cartilage of the Z joint. The capsules have a rich sensory innervation, consisting of mechanoreceptors for proprioception and free nerve endings containing substance P for nociception (Giles and Taylor, 1987; Yamashita et al., 1996). However, they have a poor blood supply, which slows the healing of these structures once they are damaged (Giles, 1992b). The multifidus lumborum muscle attaches to the articular capsule, which lies just medial to the primary attachment of this muscle to the mamillary process. The multifidus lumborum muscle may put tension on the capsule and help keep it from being entrapped in the joint space (Taylor and Twomey, 1986).
The posterior and lateral aspect of each lumbar inferior articular process (IAP) has a “lip” that projects further posteriorly than the medial aspect of the IAP, which is more anteriorly located. Consequently, the articular capsule “wraps around” this posterior lip of the lateral aspect of the IAP before attaching to the more anteriorly positioned medial aspect of the IAP. The cervical and thoracic IAPs are oriented differently and do not have this posterior lip; consequently, their capsules do not have a wrap-around component. Boszczyk et al. (2001) found that this wrap-around region of the lumbar Z joint capsule was thicker and more fibrocartilaginous in nature (containing type II collagen, aggrecan, and link protein) than the thoracic Z joint capsules, which were found to be thinner and more purely fibrous (rather than fibrocartilaginous) in nature. The entheses (attachment sites) of the lumbar Z joint capsules to the lumbar inferior and superior articular processes were found to have the same fibrocartilaginous make-up as the wrap-around portion, indicating that traction forces were placed on the entheses. The authors believed that the costovertebral (costocorporeal) and costotransverse articulations of the thoracic region, along with the spatial orientation of the thoracic articular processes, spared the thoracic capsules from the traction and compressive forces placed on the lumbar Z joint capsules (Boszczyk et al., 2001).
A detailed description of the fiber direction of the outer part of the lumbar Z joint capsules and the clinical significance of the fiber direction in the lumbar capsule is given in Chapter 7.
The articular capsules are thinner superiorly and inferiorly, where they form capsular recesses that cover fat-filled synovial pads. Defects exist within the superior and inferior aspects of the joint capsule and allow for the passage of small nerves and vessels. The synovial joint recesses and the development of synovial joint cysts are discussed in further detail with the lumbar region, where they have been studied the most extensively (see Chapter 7). Also, the specific innervation of the Z joint capsule by the medial branch of the posterior primary division (dorsal ramus) is discussed in Chapter 2.
Synovial Membrane
The synovial membrane, synovium, or joint lining is a condensation of connective tissue that covers the inner surface of the fibrous capsule, thus forming a sac that encloses the joint cavity (Fig. 14-7). Therefore the region of a diarthrodial joint surrounded by a synovium is known as the synovial, or joint, cavity. The synovium covers the nonarticular bone enclosed within the joint capsule and courses to the margin of the articular cartilage, where a transition zone exists between the synovium and articular cartilage. The synovium does not cover the load-bearing surface of the cartilage. The joint cavity normally contains a small amount of a highly viscous, hyaluronic acid-rich fluid that lubricates the joint surfaces. This fluid is known as synovial fluid and is produced by the cells within the synovial membrane (see Synoviocytes). The major function of the synovial membrane is to produce synovial fluid. Another function is to absorb waste products of metabolism and cellular debris before they can accumulate in the Z joint cavity.
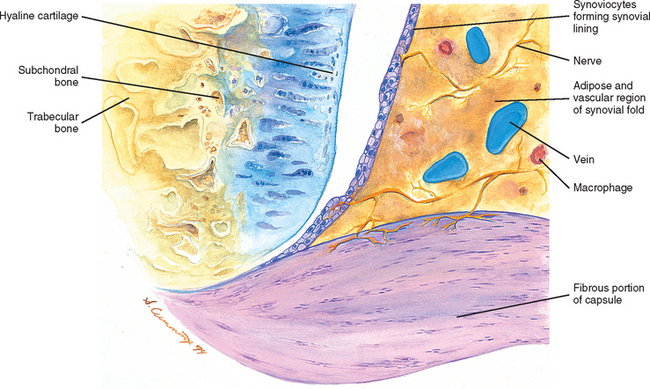
FIG. 14-7 Portion of the zygoapophysial (Z) joint at a magnification of approximately 40×. The region here is shown by the box in Figure. 14-1. Portions of the articular cartilage, subchondral bone of the superior articular process and mamillary process, the articular capsule, and the Z joint synovial fold can be seen.
Typically projections of the synovial layer extend into the synovial cavity as Z joint synovial folds (Giles, 1992a). Their purpose is to fill in the small gaps along the periphery of the joint, where the articular cartilages of the opposing surfaces do not normally come in contact with one another. These folds also produce synovial fluid and provide an efficient mechanism for the distribution of this fluid directly into the joint cavity.
Z joint synovial folds contain a relatively large amount of adipose tissue at the region of their attachment to the fibrous layer of the articular capsule. They possess a nociceptive sensory nerve supply of free nerve endings containing substance P (Giles, 1987), and at times they may extend a considerable distance into the joint, in which case their central tips usually are fibrous. Entrapment of these folds between or extrapment of them peripheral to the articular surfaces of the Z joint have been implicated as possible causes of back pain (Mooney and Robertson, 1976; Giles and Taylor, 1987; Bogduk and Twomey, 1991). Giles (1992a) also states that traumatic synovitis of these folds may cause the release of pain-mediating agents and subsequent back pain.
Synoviocytes
Transmission electron microscopy studies reveal that a discontinuous layer of cells, known as synoviocytes, lines the free surface of the synovial membrane. Although synoviocytes resemble other connective tissue cells, they differ from ordinary fibroblasts (see Table 14-1) in their ultrastructural features and metabolic activities.
Synoviocytes have been classified into two types based on their cellular morphologic structure: fibroblast-like cells, or type A synoviocytes, and type B synoviocytes. Type A synoviocytes are somewhat numerous and are characterized by the presence of abundant cytoplasmic organelles such as endoplasmic reticulum. These cells are involved in secretion and are believed to synthesize hyaluronic acid and glycoproteins (see following discussion). Type B synoviocytes are similar to macrophages and are involved in phagocytosis. Types A and B synoviocytes are not connected by junctional complexes and do not rest on a basement membrane; therefore they do not constitute an epithelial lining of the joint cavity, although they do create a smooth secreting surface for the synovium. Small folds of synovium, or synovial villi, can be found periodically along the surface of the synovial membrane.
The synovial fluid produced by the type A synoviocytes is rich in hyaluronic acid and also contains protein, although its protein content is less than that of blood plasma (Triano, 1992; Williams et al., 1995). The hyaluronic acid imparts synovial fluid with great viscosity. Coiling of the hyaluronic acid molecules and interlocking between different molecules allow the synovial fluid to act as a shock absorber during compressive loads. However, during shear forces the coiled hyaluronic acid molecules straighten and the interlocking between molecules decreases, resulting in smooth, low-friction movement between the adjacent Z joint surfaces.
Supporting Cells and Extracellular Matrix of Connective Tissue: Functional Components
Mature Connective Tissue.
Cells of connective tissue.
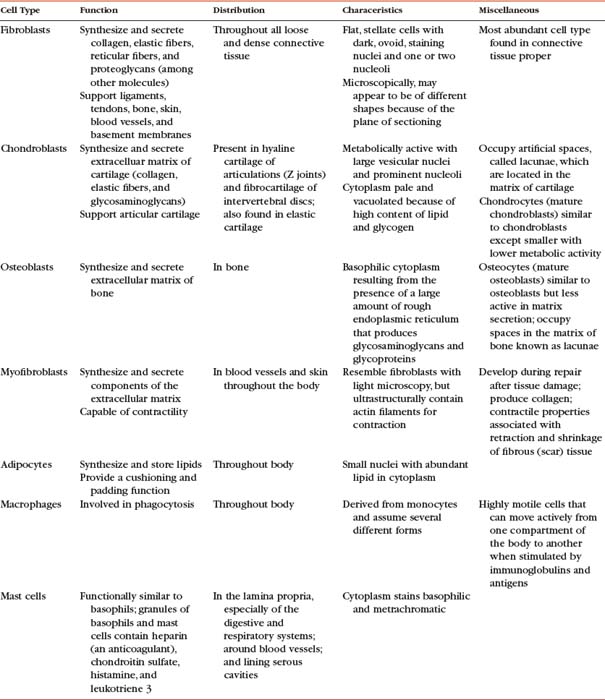
As mentioned, connective tissue consists of cells, fibers, and ground substance. The type of supportive resident cells found in connective tissue varies considerably and may include fibroblasts, chondroblasts and chondrocytes, and osteoblasts, osteoclasts, and osteocytes. These cells are important when considering the connective tissue of spinal structures. Adipocytes, mast cells, macrophages, and myofibroblasts are also found in connective tissue in various parts of the body. The functions and primary characteristics of these cells are listed in Table 14-1.
In addition to the fixed or resident cells of connective tissue described in Table 14-1, connective tissue also contains transient or immigrant cells. These include all the formed cellular elements found in blood with the exception of erythrocytes. The immigrant cells include the neutrophils, eosinophils, basophils, monocytes, lymphocytes, and plasma cells. When inflammation occurs, these immigrant cells leave the circulation and join fibroblasts and other connective tissue resident cells, such as macrophages. Once in the connective tissue, they fight microorganisms that cause inflammation and clean up (phagocytize) the debris that results from this process.
Collagen Synthesis.
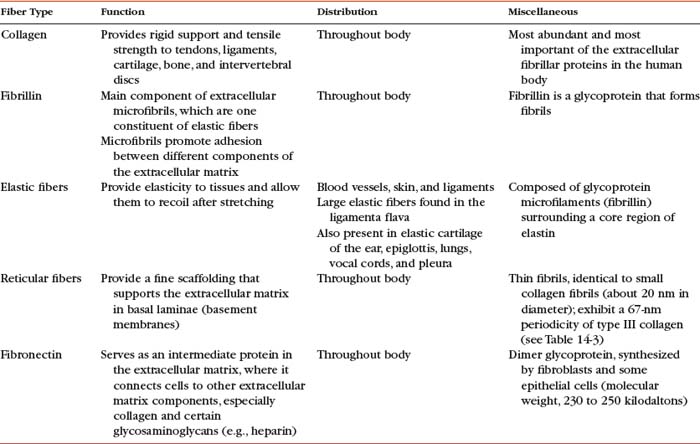
Collagen is the most important fiber type of connective tissue. Collagen is a major component of connective tissue proper, cartilage, and bone. Collagen fibers are found in abundance throughout the articular capsule and hyaline cartilage of the Z joints and also throughout the IVDs. Collagen fibers are composed of collagen macromolecules, which are the most abundant protein in the human body. Collagen fibers are flexible and strong, and they are made up of a bundle of fine, threadlike subunits called collagen fibrils. Collagen is a stable protein under the physiologic conditions that exist in connective tissue; however, collagen is constantly being degraded and replenished by collagen-secreting cells.
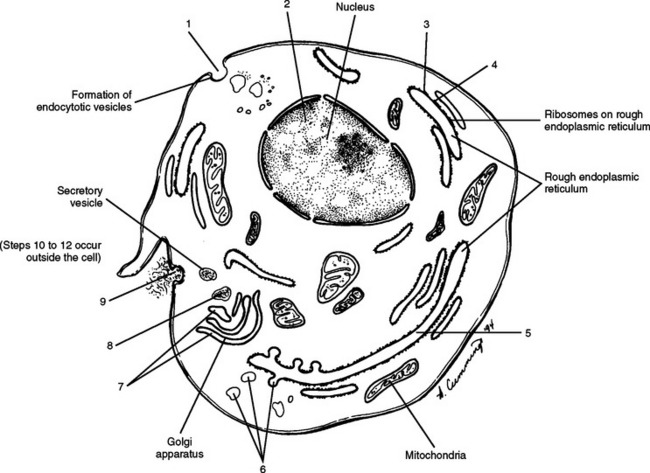
It was believed for many years that collagen synthesis occurred primarily in fibroblasts, chondroblasts, osteoblasts, and odontoblasts; however, recent investigations in collagen biology indicate that many other cell types produce this unique protein. Collagen synthesis has been studied extensively in fibroblasts (Williams et al., 1995). Fibroblasts have the extensive rough endoplasmic reticulum and well-developed Golgi apparatus required of cells actively involved in protein synthesis. Labeled amino acids endocytosed by fibroblasts can be followed autoradiographically to the rough endoplasmic reticulum (rER), later to the Golgi complex, then to the outside of the fibroblast, and eventually to the newly formed collagen fibers. This evidence indicates that the collagen synthesis pathway is similar to that of other proteins. Fibroblasts synthesize collagen de novo and secrete it into the extracellular matrix. Fibroblasts also have the ability to break down collagen with specific degradative enzymes called collagenases.
Collagen synthesis begins inside cells. However, the final processing and assembly into fibers takes place after collagen building blocks have been secreted outside the manufacturing cells. The intracellular events include synthesis of proalpha chains in the rER, hydroxylation and glycosylation of proalpha chains into triple helices in the Golgi apparatus, and formation of secretory granules (vesicles). The extracellular events include cleavage of extension peptides, fibrillogenesis and cross-linking, and assembly of fibrils into mature fibers (Fig. 14-8). Box 14-1 shows the events (steps) involved in collagen synthesis within the fibroblast (steps 1 through 9) and outside the fibroblast in the extracellular matrix (steps 10 through 12).
BOX 14-1 COLLAGEN SYNTHESIS
| 1. Uptake of amino acids via endocytosis |
| ↓ |
| 2. Formation of messenger ribonucleic acid |
| ↓ |
| 3. Synthesis, by ribosome, of alpha chains with peptides |
| ↓ |
| 4. Hydroxylation of amino acids |
| ↓ |
| 5. Glycosylation of specific hydroxyl-1 residues in rough endoplasmic reticulum (rER) |
| ↓ |
| 6. Formation of procollagen in rER and movement into transfer vesicles |
| ↓ |
| 7. Packaging of the procollagen by the Golgi complex into secretory vesicles |
| ↓ |
| 8. Movement of vesicles to plasma membrane assisted by microfilaments and microtubules |
| ↓ |
| 9. Exocytosis of procollagen |
| ↓ |
| 10. Cleavage of procollagen to form tropocollagen |
| ↓ |
| 11. Polymerization of tropocollagen into collagen microfibril |
| ↓ |
| 12. Polymerization of collagen microfibrils into a complex, 1- to 12-μm collagen fiber |
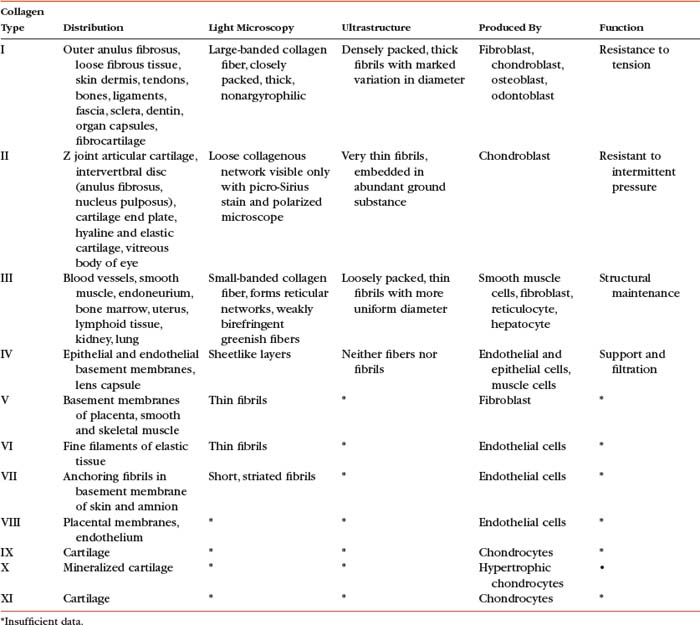
The amino acid composition of collagen is one of the features that makes collagen such a unique protein. Four amino acids compose most of the polypeptides in the collagen macromolecules. The principal amino acids that make up collagen are glycine (35%), proline (12%), hydroxyproline (10%), and alanine (11%). In the cytoplasm of the fibroblast, approximately 250 to 300 amino acids are combined by polyribosomes associated with rER to form a polypeptide with a molecular weight of 30, 000 Da. This step of translation is performed under the control of messenger ribosomal ribonucleic acid (mRNA). Three polypeptide chains are combined into polypeptide alpha triple helices with a molecular weight of approximately 100, 000 Da. These triple helices are released into the cisternae of rER (see Box 14-1, steps 1 through 3). Glycine is the third amino acid in each alpha chain of the newly formed triple helix. The amino acid after glycine frequently is proline, and the amino acid preceding the glycine frequently is hydroxyproline. Differences in the chemical structure of the alpha chains are responsible for at least 19 different types of collagen identified to date (Ross et al., 2003). Specifics of the 11 most common types of collagen can be found in Table 14-3.
Several modifications of the polypeptide chains occur within the cisternae of rER and the Golgi apparatus (see Box 14-1, steps 4 to 6 and Fig. 14-8). Disulfide bonds are formed within each polypeptide chain and between adjacent chains. Vitamin C is necessary for the formation of the disulfide bonds, and its absence results in certain types of collagen-related diseases such as scurvy. This bonding gives shape and stability to the triple-helix collagen macromolecule. The structure formed now constitutes a procollagen molecule. The procollagen molecule moves to the exterior of the cell via secretory granules (see Box 14-1, steps 7 to 9 and Fig. 14-8). Further modifications are made outside the cell. For example, enzymes cleave most of the uncoiled amino acids, thereby converting procollagen to tropocollagen molecules. These eventually aggregate to produce collagen fibrils (see Box 14-1, steps 10 to 12). Cross-links between lysine and hydroxylysine are then formed, giving the molecule its tensile strength. Changes in collagen cross-links have been seen in IVD degeneration (Duance et al., 1998).
The tropocollagen molecules are 300 nm long and 1.5 nm in diameter. They consist of three polypeptide chains that are twisted around one another to form a right-handed superhelix with a head and a tail end. Numerous tropocollagen molecules lie end-to-end and also in parallel chains or rows. All the molecules face the same direction, and approximately one fourth of the length of the tropocollagen molecule overlaps between the parallel rows. Therefore a tropocollagen molecule of one row ends approximately one fourth of the distance along the length of another tropocollagen molecule of an adjacent row. This configuration results in a regular 64- to 67-nm periodicity that is clearly visible on an electron micrograph. Figure 14-9 shows collagen fibers within the IVD.
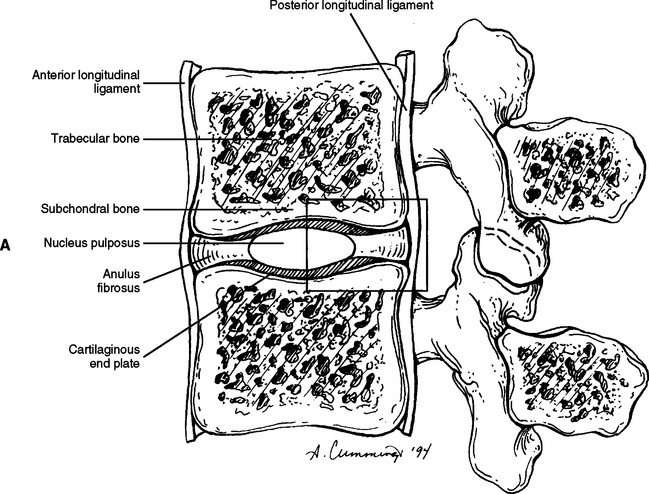
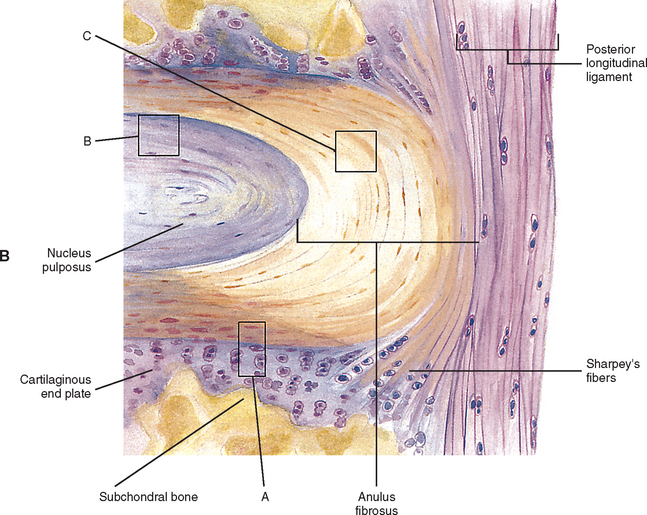
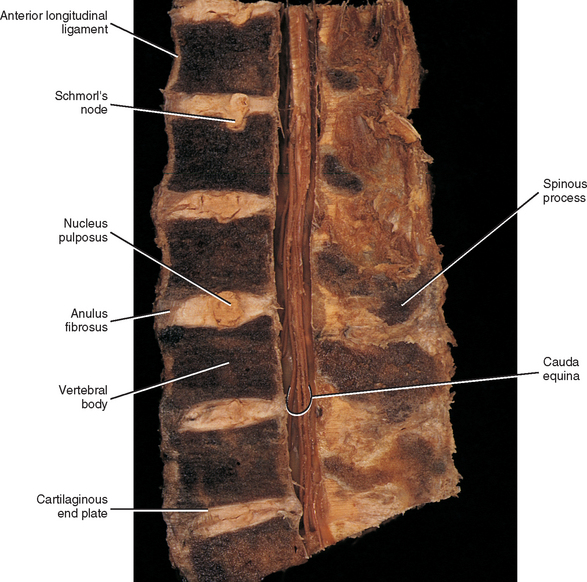
FIG. 14-9 A, Sagittal section of two adjacent vertebrae and the intervertebral disc between them. B, Shows the boxed region in A at higher magnification (magnification ≈ ×15). In addition to showing the anulus fibrosus (AF), nucleus pulposus, and cartilaginous end plate, the vertebral body and posterior longitudinal ligament are shown. Notice that the outer fibers of the AF attach to the cortical and subchondral bone of the vertebral body. These attachment sites are known as Sharpey’s fibers. The collagen fibers of the inner layers of the AF enter the end plate and curve to run parallel to the discal surface of the vertebral body. (The boxes labeled A, B, and C refer to the regions shown in Figure. 14-10.)
Types of Collagen.
At present, 19 different types of collagen have been positively identified. They are designated as types I through XIX. Types I to V are the most abundant types of collagen. Types VI to XIX are considered less important because they occur in small quantities. Several of the minor types of collagen (types VI, IX, X, XI, XII, and XIV) are present in small amounts in the IVD (Duance et al., 1998). Table 14-3 lists the characteristics of the 11 most important types of collagen.
Types I, II, and III are arranged as ropelike fibrils and are the main forms of fibrillar collagen. Type I collagen consists of two alpha-1 chains and one alpha-2 chain and represents 90% of all collagen fibers distributed in connective tissue. Because type I fibers resist tensile stresses, their orientation and cross-linking vary according to the local environment. Type I collagen is found in bone, tendon, and the anulus fibrosus (AF) of the IVD. It is also found in the skin and cornea (see Table 14-3).
Type II collagen fibers are small, banded fibrils averaging 20 nm in diameter. They help to form the extracellular matrix of hyaline cartilage, including that of the Z joints and cartilaginous end plates (CEPs) of the IVDs. Type II collagen is the main type of collagen found in the nucleus pulposus (NP) of the IVD. It is also found in elastic cartilage and the cornea and vitreous body of the eye. These fibers demonstrate a high electrostatic attraction for the chondroitin sulfate glycosaminoglycans. Type II collagen contains a higher degree of lysine hydroxylation than type I collagen.
Types III and IV collagen are well distributed throughout the body but are not found to any great extent in Z joints, IVDs, or other spinal tissues, although type III has been found in regions adjacent to spondylosis (Schollmeier, Lahr-Eigen, and Lewandrowski, 2000). The key features of these fibers and collagen types V through XI are listed in Table 14-3.
Ground Substance.
The cells and fibers of connective tissue are surrounded by a translucent, fluidic, homogeneous, gel-like matrix called amorphous ground substance (Bloom and Fawcett, 1986). The ground substance exhibits no structural organization that is visible with light microscopy. Extracellular amorphous ground substance plays a vital role in the regulation of tissue nutrition, support, and maintenance of proper water content. Based on chemical analysis, the extracellular ground substance of connective tissue has the physical properties of a viscous solution or thin gel and consists of proteoglycans and glycosaminoglycans of various types. Proteoglycans and glycosaminoglycans are an important part of the hyaline cartilage of the Z joints and the cartilaginous (vertebral) end plates of the IVDs. They are also being studied with regard to the AF and NP of the IVD. Therefore glycosaminoglycans and proteoglycans are discussed in further detail with the articular cartilage of the Z joint (see previous discussion) and with the IVD (see following discussion).
MICROSCOPIC AND MOLECULAR STRUCTURE OF THE INTERVERTEBRAL DISCS
Symphyseal joints unite the vertebral bodies, and these joints are made up of the IVDs. The IVDs permit a limited amount of movement between the vertebral bodies while maintaining a union of great strength. The intrinsic stability of the motion segment (two adjacent vertebrae and the ligaments, including the disc, between them), and therefore of the whole spine, results mainly from the IVDs and the ligaments associated with them (Bogduk, 1997). The paraspinal and trunk muscles provide the spine’s extrinsic stability.
IVDs (see Fig. 14-9) are important parts of the spinal column and play an active and important role in the spine’s physiologic function. The physical properties, elasticity, and resiliency of the IVDs allow them to give support to the spine and allow motion to occur between adjacent vertebral segments, while also preventing too much motion from occurring between the same segments. The IVDs also allow the spine to return back to its original shape after being compressed or stretched (Chai Ben-Fu and Tang Xue-Ming, 1987).
The IVD consists of three main parts: the outer AF, which consists of a series of fibrocartilaginous rings (except in the cervical region, where it is a solid, crescent-shaped, fibrocartilaginous structure); the inner gelatinous NP; and the CEPs of hyaline-like cartilage. The end plates are located between the bony vertebrae and other parts of the IVD (Ghosh, 1990).
Each IVD is reinforced peripherally by circumferential ligaments (see Fig. 14-9, A). A thick anterior longitudinal ligament extends down the anterior aspect of the spinal column and is attached to the vertebral end plates. It provides additional anterior support to the AF. A thinner posterior longitudinal ligament spans across the posterior aspect of each disc and is firmly attached to the IVD’s posterior aspect.
The IVD is specialized connective tissue designed to provide strength, mobility, and resistance to strain. All three parts of the IVD listed previously (Fig. 14-10; see also Fig. 14-9, B) consist of water, cells, proteoglycans (PGs), and collagen. These components are found in varied concentrations in the three different regions of the disc. In fact, the varied concentrations of these basic components within the IVD make it a specialized type of connective tissue. For example, after an early age (≈2 years) the disc has no blood supply (except for the vessels within the vertebral bodies that are adjacent to the CEPs and remain until 11 to 12 years of age), and so receives its nutrition from the adjacent vertebral bodies. The PGs are essential in the process of attracting fluid and nutrition to the IVD from the adjacent vertebral bodies. The PGs are negatively charged and attract Na+, which then attracts water and other nutrients by osmotic flow. The PGs have been found to actually regulate the amount and type of molecules entering the IVD. Breakdown of PG molecules in the IVD has been associated with the decreased fluid and tissue breakdown found in disc degeneration. Because chondroitin sulfate is a major component of the PGs, this substance and a related molecule, glucosamine sulfate, are frequently given as part of the conservative treatment of disc degeneration. Therefore PGs are important to the health and treatment of the IVD.
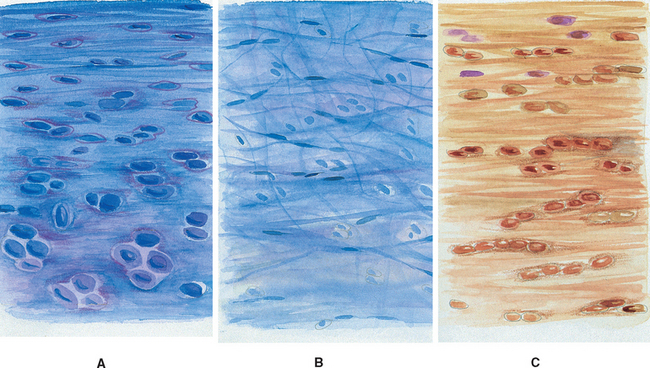
FIG. 14-10 A, Regions of the intervertebral disc. A, B, and C correspond to lettered boxes of Figure. 14-9, B. A, Cartilaginous end plate. B, Nucleus pulposus. C, Anulus fibrosus. (A, B, and C represent a magnification ≈ ×100)
Collagen is another of the important components of the IVDs. As mentioned, the main type of collagen in the AF is type I, and is a ropelike molecule that is tough and strong and gives the AF its ability to withstand the forces and loads placed on the IVD. The NP has a higher concentration of the less-well-organized type II collagen.
Therefore each of the building blocks of the IVD is important and clinically relevant. In addition, each of the four constituents is closely related to the others. For example, the collagen fibers within the IVD become taut during movements of the spine and tend to restrain the PGs. The PGs in turn allow the IVD to deform. Because of its ability to absorb fluid (swell) and then to maintain its hydration (water), the PG gel of the NP is able to resist compression under large external loads (Weiss, 1988). The cells in turn maintain the proper levels of PGs and collagen fibers. Therefore the IVD is able to act as a lubricating cushion that prevents adjacent vertebrae from being eroded by abrasive forces during movement of the spinal column. The hydrated gelatinous NP serves to a certain extent as a shock absorber to reduce the impact between adjoining vertebrae (Junqueira and Carneiro, 2003), although the vertebral body helps in this function as well.
The histologic changes that take place in the IVD with advancing age have been described in postmortem studies by several investigators (Brown, 1971; Pritzker, 1977; Roberts et al., 1989, 1996; Boos et al., 2002). These changes include loss of distinction between the NP and AF, desiccation and fibrosis of the NP with fibrillation of the matrix, brown discoloration of the nucleus, fissuring of the nucleus and AF, fractures of the vertebral end plate, and osteophyte formation. Figure 14-11 demonstrates a series of events associated with degeneration of the IVD. Based on plain x-ray films, the fundamental diagnostic features of disc degeneration are reported to be disc space narrowing and osteophytosis. Decreased hydration as demonstrated by a decreased signal intensity of the IVD on T1- and T2-weighted magnetic resonance imaging (MRI) scans is also an indication of IVD degeneration. The section entitled Normal Aging of the Intervertebral Discs and Intervertebral Disc Degeneration discusses the changes of IVD degeneration in further detail. In addition, Chapter 7 describes the consequences of these changes and the development of internal disc disruption. Chapter 2 describes the gross anatomic features of the IVD and the clinical relevance of these features, and Chapter 11 discusses IVD bulging, protrusion, and extrusion and their effects on the neural elements within the vertebral canal (i.e., cauda equina and dorsal root ganglia).
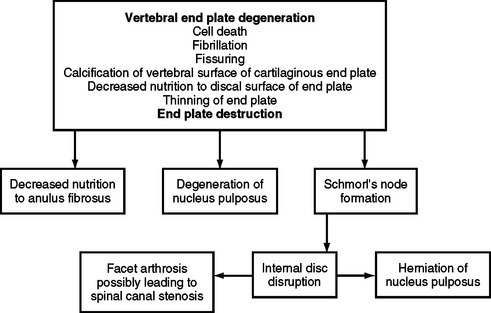
FIG. 14-11 Flowchart demonstrating a series of events leading to degeneration of the intervertebral disc.
Anulus Fibrosus
The AF is the rigid, outer series of rings (lamellae) that forms the peripheral portion of the IVD (Fig. 14-12 and Fig. 14-13). It functions to absorb pressure from the central well-hydrated (jellylike) NP. The tightly packed collagen fibers of the AF normally do not allow the large PG molecules of the NP to pass between them, even when the IVD is subjected to large compressive forces. The adult AF is not distinctly separated from the NP or cartilage of the vertebral end plates (Inerot and Axelsson, 1991).
The histologic features of the AF do not change much from childhood to maturity. The outer ring of the AF consists of an external tough layer of dense collagenous connective tissue, whereas the remainder of the AF is primarily composed of overlapping concentric layers of fibrocartilage. The outer part of the AF attaches to the margins of adjacent CEPs in infancy and childhood and to the outer rims of adjacent vertebral bodies in adolescence (see Fig. 14-9, B, Sharpey’s fibers).
Light and electron microscopy indicate that a typical lumbar AF is composed of fibrocartilage and has a lamellar structure. Anteriorly the AF consists of more than 20 moderately thick lamellae. The outer lamellae are entirely fibrous and contain thick, tightly packed bundles of type I collagen fibers (Ghosh, 1990; Schollmeier, Lahr-Eigen, and Lewandrowski, 2000). Although the outer AF is composed of type I collagen (see Table 14-3), the fibers of the inner AF are composed of type II collagen (Bishop, 1992; Schollmeier, Lahr-Eigen, and Lewandrowski, 2000). The lamellae of the inner part of the AF also have a richer PG ground substance associated with them, which increases the capacity to resist compression (McDevitt, 1988). The collagen fibers in each lamella are orientated parallel to one another and form an angle of inclination (≈25 to 30 degrees) with the horizontal axis of the bony vertebral rims. The fibers of each consecutive layer form approximately a 120- to 130-degree angle with the fibers of adjacent lamellae. The lamellar structure and the angle of inclination of the collagen fibers enable the AF to sustain the normal forces of compression, torsion, and flexion that occur during movements of the IVD (Chai Ben-Fu and Tang Xue-Ming, 1987).
As mentioned, the anterior and lateral parts of the AF are composed of more than 20 moderately thick lamellae. The outer lamellae are loosely attached to the strong anterior longitudinal ligament (Ghosh, 1990
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree