Fig. 19.1
Structural elements of the bacterial cell
Flagella
Pili and fimbriae
Capsule or slime layer
Cell wall
Cytoplasmic membrane
Cytoplasm
Spores
Cytoplasm, cytoplasmic membrane and cell wall are always present. The presence of the other components depends on the type of micro-organism, the culture conditions and the growth phase.
Flagella
Bacteria become motile by means of flagella [41]. Bacterial flagella are protein threads which originate in a defined region of the cytoplasmic membrane and protrude through the peptidoglycan layer and the outer membrane. The number of flagella per cell and their position depends on the species. Pseudomonas aeruginosa (Ps. aeruginosa) has only one (polar) flagellum at the tip of the cell, whereas Escherichia coli (E. coli) has numerous flagella spread over the entire cell surface (peritrichous). Flagella may play an important role in pathogenicity [41, 42].
Pili and Fimbriae
The surface of cells of some bacterial species is covered with many (10 to several thousands), thin (3–25 nm), and long (up to 12 μm) threads called pili, or fimbriae. They play a role in the initial adhesion of bacteria to host tissues and inanimate surfaces [43, 44]. Attachment to a surface is the first step in biofilm formation. Upon attachment on tissue cells they may trigger a number of biochemical signals from the host, which ultimately leads to the bacterial disease [45].
Capsule or Slime Layer
Capsules and slime layers – collectively called glycocalix – consist of source polysaccharide material secreted by the cell. A capsule is a rigid structure, whereas a slime layer, or loose extracellular slime, is more flexible, with diffuse boundaries. The glycocalix has several functions. It is involved in cell attachment and it may protect cells from being digested, a phenomenon known as phagocytosis. Whilst encapsulated strains of Streptococcus pneumoniae are highly pathogenic, non-encapsulated mutants are completely avirulent [46, 47]. Dextran, a slime layer product of Leuconostoc mesenteroides of relatively low molecular weight can be used as a therapeutic agent in restoring blood volume [48].
Cell Wall Constituents
The outer surface of the bacterial cell plays an important role in the adhesion of the cell to various surfaces. In addition to the factors that have been discussed, adhesion may also be mediated by so-called surface-associated adherence factors, usually designated as adhesins. Adhesion, which is the first step in a series of events leading to colonisation, biofilm formation and ultimately infection, is a specific process in which the adhesin “recognises” a receptor on the host surface. This specificity explains why micro-organisms such as Influenza or Mycobacterium tuberculosis can cause targeted infection of the respiratory tract but otherwise are relatively harmless when contacting other host tissues.
The cell wall gives the cell its shape and strength. The cell wall must resist the internal osmotic pressure of the cell that is estimated to be about 2 bar. The composition of cell walls of gram-positive bacteria is very different from those that stain gram-negative.
Gram-Positive Cell Wall
Peptidoglycan is the common cell wall component of bacteria (excluding mollicutes) and gives the wall its shape and strength. It is a polymer consisting of a backbone of alternating N-acetylglucosamine and N-acetylmuramic acid residues, cross-linked with small peptide bridges. Peptidoglycan accounts for about 80–90 % of the wall of gram-positive bacteria and for about 10 % of the gram-negative cell wall.
Gram-Negative Cell Wall (Outer Envelope)
The gram-negative cell wall contains only a shallow peptidoglycan layer. On the outer side of this layer is the outer membrane, a complex structure consisting of four major components: phospholipids, lipopolysaccharide, proteins (e.g., porins), and lipoprotein. Lipolysaccharide (endotoxin) is responsible for the pyrogenicity of the gram-negative bacteria.
Cytoplasmic Membrane
The cytoplasmic membrane, or plasma membrane is a phospholipid bilayer into which proteins/enzymes are embedded. The function of the cytoplasmic membrane is to act as a selective permeability barrier between the cytoplasm and the exterior environment. A mesosome is an organelle of bacteria that appears as an invagination of the plasma membrane and functions either in DNA replication and cell division, energy production, or excretion of exoenzymes. Flagella (if present) originate in a special structure in the cytoplasmic membrane (see the section on Flagella under Structure of the Bacterial Cell).
Cytoplasm
The cytoplasm is a viscous liquid, which contains all other essential elements for the living cell. The genetic material is mainly organised in the genome, a circular string of DNA. There is no discrete bacterial nucleus. The genetic code is translated into messenger RNA and then transported to the ribosomes, where the protein synthesis occurs. The building blocks of the proteins (amino acids) are transported to the ribosomes by means of transfer RNA.
Some genetic information such as antibiotic resistance may be encoded in plasmids – DNA molecules that are independent of the genome and that can replicate themselves. Some plasmids contain a set of genes (in the tra region) that enable the transfer of the plasmid by cell to cell contact (conjugation). The plasmid is replicated during this process and its genetic information (e.g. antibiotic resistance) is thus transferred to the recipient cell. There are both intra-species and inter-species plasmid transfer phenomena. The cytoplasm may also contain reserve material such as polyhydroxybutyric acid, and other substances of uncertain function (e.g. polyphosphate, volutin).
19.3.3.4 Bacterial Endospores
Some gram-positive rods such as the genera Bacillus, Geobacillus and Clostridium are capable of forming endospores that enable these genera to survive harsher conditions, such as exposure to heat, radiation, or chemicals. Bacterial spores are resistant forms of life. Some experts have suggested that they may remain viable (capable of life) for millions of years.
The bacterial spore has a complex structure, consisting of various layers, including coat and cortex, all of which play a role in long-term survival (see Fig. 19.2).
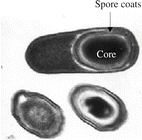

Fig. 19.2
The bacterial spore. © 2003 Ricca and Cutting, Journal of Nanobiotechnology 2003, 1:6, doi:10.1186/1477-3155-1-6, published by BioMed Central
Endospore formation is a non-reproductive process: one cell produces only one spore which, after germination, produces one vegetative cell.
Bacterial sporulation occurs when growth decreases due to exhaustion of an essential nutrient. It is a complicated process requiring the participation of more than 200 enzymes. Conversion to a vegetative cell involves three steps: activation, germination and outgrowth. Activation can be accomplished by heating the spores to a non-lethal temperature. Germination can be induced by a variety of events, including exposure to nutrients (amino acids, sugars, or purine nucleosides), non-nutrient germinants (dodecylamine, lysozyme) and heating [49]. The spore loses its characteristic constituents, and heat resistance decreases dramatically. In the last stage water is taken up, and metabolism (synthesis of ATP, proteins and genetic material) resumes. Heat activation is an important factor in the occurrence of a shoulder in the survival curve of bacterial spores upon heating.
Destruction of bacterial spores is the ultimate goal of sterilisation processes. Bacterial spores are typically used in biological indicators for validation and monitoring of sterilisation processes.
19.3.4 Endotoxins/Pyrogens
Pyrogens are substances that cause a febrile reaction. Two groups of pyrogens can be distinguished: exogenous and endogenous pyrogens. The exogenous pyrogens form a heterogeneous group of substances; the most important one is lipopolysaccharide (LPS) from the cell wall of gram-negative bacteria. LPS, also known as endotoxin, has antigenic properties (O antigen) and causes fever when injected intravenously. Lipoteichoic acid, muramyldipeptide, porins, glycans and nucleic acids, are examples of non-endotoxin pyrogens originating from bacteria (gram-positive and gram-negative), yeast and moulds.
The pyrogenic activity of LPS is much higher than that of most other pyrogenic substances. This is the reason why an in-vitro limit test for LPS (the Limulus Amoebocyte Lysate, or LAL test) generally suffices for quality control purposes of parenteral medicines and raw materials, including water for injection.
The European Pharmacopoeia requires the rabbit pyrogen test for a number of vaccines, some antibiotics, and specific excipients including glucose, if intended for the preparation of large volume parenterals (see Sect. 32.8). These products may be contaminated with pyrogens other than LPS, or are known to inhibit the LAL test.
A third test, the monocyte activation test (MAT) is based on the in-vitro activation of human blood cells by pyrogens. This leads to the release of pro-inflammatory cytokines tumour necrosis factor alfa (TNF-alfa), interleukin-1 beta (IL-1beta) and interleukin-6 (IL-6) that are determined by Enzyme-Linked Immuno Sorbent Assay (ELISA). Consequently, the MAT will detect the presence of both exogenous and endogenous pyrogens in the test sample. The MAT is suitable, after a product-specific validation, as a replacement for the rabbit pyrogen test [50].
The reagent for the LAL is isolated from the blood of the horseshoe crab (Limulus polyphemus). The blood is collected from wild animals. Many animals do not survive (mortality rates of up to 30–50 % have been reported), and this living fossil is threatened with extinction. It is to be expected that in the near future the MAT test or other alternatives for the LAL test and the rabbit test will be more generally introduced. The development of such new methods will significantly reduce animal testing. The commercially most successful alternative method, which replaces the rabbit pyrogen test for bacterial impurities in medicines with a test using human cells, could save the life of 200,000 rabbits a year.
19.3.5 Biofilms
Biofilms are multicellular, microbial communities held together by a self-produced extracellular matrix that adhere to biological or non-biological surfaces [51]. A biofilm has a defined architecture, and it provides an optimal environment for the exchange of genetic material between cells, e.g. spread of antibiotic resistance. Cells within a biofilm may communicate via quorum sensing (see Sect. 19.1.7), which may in turn affect biofilm processes such as detachment of cells. The ability to form biofilms is a universal attribute of bacteria and many other micro-organisms.
Elimination of bacteria in this mode of growth is challenging due to the resistance of biofilm structures to both antimicrobials and host defences.
Biofilms have great importance for public health because of their role in certain infectious diseases and their role in a variety of device-related infections. Biofilm infections on indwelling devices or implants are difficult to eradicate because of their much better protection against macrophages and antibiotics, compared to free living cells, leading to severe clinical complications often with lethal outcome.
19.3.6 Fungi (Moulds and Yeasts)
Fungi are widespread in nature and have considerable economic and medical importance, because:
They may contaminate and cause spoilage and deterioration of pharmaceutical preparations. Mould and yeasts are the second cause of FDA recalls in non-sterile pharmaceuticals [52].
This group of organisms is used by producers of active substances, including antibiotics, such as penicillins by Penicillium species, or alkaloids, such as ergotamine by Claviceps purpurea.
Some fungi are pathogenic to humans. They may cause infections (e.g., Trychophyton sp. or Candida sp.), or produce toxic substances (e.g., aflatoxin by Aspergillus flavus).
Two groups of fungi are relevant in the context of pharmaceutical products or processes: the moulds and the yeasts. Their physical differentiation is not always clear, because some fungal species (e.g., Candida, Histoplasma and Cryptococcus) show dimorphism, a phenomenon in which a filamentous and a yeast-like stage both exist.
Moulds are obligate aerobic micro-organisms; they grow on the surface or in the uppermost layers of the substrate. Characteristic of moulds is the filamentous body, the mycelium. Vegetative growth of moulds occurs at the tip of the individual filaments (hyphae).
Depending on the species, hyphae may be divided into compartments by means of septa (Eumycetes). Each septum contains a pore, which allows flow of cytoplasmic constituents from one compartment to another. The lower fungi (Phycomycetes) have aseptate (coenocytic) hyphae; the mycelium is a multinucleated cell.
Asexual reproduction of moulds normally occurs by means of spore formation. From the mycelium special branches reach up into the air. At the tip of these conidiophores the spores (conidiospores) are formed on a genus specific structure. The colour of mould colonies on solid substrates (e.g., different shades of green for Penicillium species, or black for Aspergillus niger) is entirely due to the massive production of these conidiospores.
Mould spores may cause significant issues in the production of pharmaceutical preparations since they survive desiccation and may be transported via air, personnel or material flow into products.
The spores are readily dispersed into the environment and may form a new mycelium. Because of mechanical forces, such as those exerted during vortexing, hyphae may break up into smaller fragments, which may also form new mycelia. Clumps of conidiospores may also break up into smaller units. Such fragmentation caused by vigorous mixing in the course of microbiological examination of pharmaceutical samples may lead to considerable uncertainty in fungal counts.
Yeasts are typically unicellular organisms. Yeast cells are spherical or oval. Growth (asexual reproduction) takes place by a process called budding. A new cell is formed as an outgrowth of the mother cell, the daughter cell enlarges and finally the two cells separate. Pathogenic dimorphic fungi usually form yeast-like cells in the human body and a mycelium at room temperature (e.g. Histoplasma). Candida sp. is an exception because it forms hyphae in the host tissue.
Sexual reproduction is associated with many yeasts and moulds. A stage in which spores are formed is always involved in the sexual process. Depending on the type of sexual spore formation four groups of moulds can be distinguished: Ascomycetes, Basidiomycetes, Zygomycetes, and Oomycetes. The spores are called ascospores, basidiospores, zygospores, and oospores, respectively.
Fungi for which no sexual reproduction has been demonstrated are classified as fungi imperfecti (Deuteromycetes). The majority of fungi thus far classified fall into this category. Penicillium and some Aspergillus species are well-known representatives of this group.
The cell wall of fungi consists of 80–90 % polysaccharides. Chitin is a common constituent of fungal cell walls, but is replaced by other substances such as mannan, galactosan or chitosan in some species. Peptidoglycan, the common constituent of bacterial cell walls is never present. This phenomenon explains why fungi are insensitive to antibiotics that inhibit murein synthesis, such as the penicillins and the cephalosporins. Sterols are essential structural components of the fungal cytoplasmic membrane. This characteristic makes fungi sensitive to antibiotics that interact with sterols, such as nystatin and amphotericin.
19.4 Fate of Micro-organisms in Pharmaceutical Preparations
In the previous sections the characteristics of potential contaminants of pharmaceutical preparations and their requirements for survival and growth have been discussed. There appears to be a complicated interplay between the type and characteristics of the micro-organisms (e.g. spore formation), their ability to form biofilms, the composition of the environment, and external factors, particularly the temperature. The fate of a micro-organism in a pharmaceutical preparation depends on this interplay. Four different curves may be observed when numbers of colony forming units (CFU) are plotted against time (Fig. 19.3).
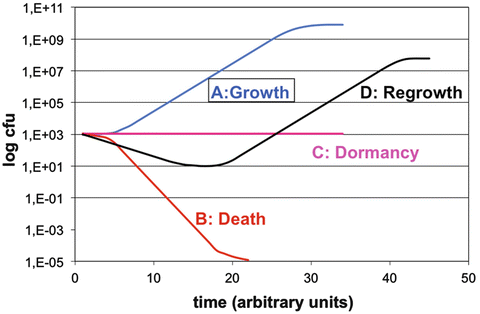

Fig. 19.3
Fate of micro-organisms in pharmaceutical preparations
Microbial growth follows the well-known sigmoid growth curve, with lag phase, log phase and maximum stationary phase. Microbial destruction under influences of heat, radiation or chemicals is frequently a first order kinetic process. When microbial destruction is plotted on a semi-logarithmic scale, a straight line is observed. A ‘shoulder’ is sometimes observed at the beginning of the curve. This lower death rate is attributed to the genetic repair mechanisms of the cells, e.g. when exposed to low doses of UV radiation. Bacterial spores must be ‘activated’ before they can germinate and grow out to become prototypical vegetative cells. This phenomenon may also cause a ‘shoulder’ in survival curves. At the end of the survival curve, a ‘tail’ may be observed, indicating the presence of resistant cells or clumps of cells. True dormancy is found only in bacterial endospores. Nevertheless, even vegetative organisms can produce an effective state of dormancy because of either a relatively slow death rate or growth and kill rates that offset each other.
In pharmaceutical preparations another type of curve is sometimes observed. An initial decrease in the number of colony forming units may occur, followed by an increase. This phenomenon can be observed when analysing data from preservative efficacy testing of inadequately preserved dosage forms. It is the reason why in pharmacopoeial preservative efficacy tests the number of viable cells must be followed for a period of 28 days (see Sect. 32.8).
19.5 Biological Contamination of Pharmaceutical Preparations
19.5.1 Impact of Microbial Contamination
Microbial contamination of pharmaceutical products may result in deterioration of the product or direct hazard to the patient.
Whether a contaminated pharmaceutical product will trigger infection or disease in the patient depends on various factors such as:
Number of micro-organisms (CFU per g or mL)
Ability of the contaminant to grow and metabolise components of the product
Properties of the particular strain
Immunocompetence of the patient, due to disease (AIDS) or use of immunosuppressiva
Route of administration
Deterioration or spoilage of the product because of microbial growth may result in several effects including:
Loss of texture because of metabolism of oil/fat phase
Loss of organoleptic quality because of production of olfactory products
Loss of preservative efficacy because of metabolism of the preservative
Loss of package integrity because of excessive gas production
Loss of therapeutic activity because of metabolism of the active substance(s)
Release of toxigenic substances, including toxins (e.g. aflatoxin) and pyrogens
19.5.2 Origin of Microbial Contamination
The contamination can be primary or secondary. Primary contamination occurs at the premises or during preparation:
Personnel. Personnel account for the majority of contaminations in the clean room environments. This can be explained by the high number of micro-organisms located on or in the human body. The organisms may be introduced into the environment due to inadequate gowning or hygiene, infrequent or ineffective hand washing and disinfection procedures, unqualified behaviour (non-clean room adequate) of personnel, etc. In the aseptic production of sterile pharmaceutical preparations living micro-organisms should not enter the aseptic filling area and the product should not contain any viable micro-organism. In those situations, low-level microbial contaminations of products occur mostly at critical interventions near to the product during processing. Microbial contamination of non-sterile pharmaceutical preparations may not originate primarily from the human body, but raw materials, equipment, air and packaging material may also play an important role
Raw materials. Raw materials from natural origin may be highly contaminated with micro-organisms especially spore-forming bacteria and moulds and in some cases with more critical Enterobacteriaceae. Soon after a publication on salmonellosis in more than 200 persons caused by the contamination of thyroid tablets with two types of Salmonella originating from the raw material [53], proposals for the examination of non-sterile pharmaceutical preparations and acceptance criteria were published [54].
Water. Water may be used to clean equipment and clean rooms as well as a product component. Water contains water-borne micro-organisms that may grow under low nutrient conditions. In a recent review of FDA product recalls, almost half (48 %) of them were due to contamination by water-borne micro-organisms such as Burkholderia cepacia, Pseudomonas species, or Ralstonia pickettii [52].
Air. Micro-organisms may be carried over from dust or soil particles and may be transported into manufacturing areas by personnel, material or airflow. Mould spores for instance were carried over from a highly contaminated source into the production room [55].
Equipment. Equipment may be contaminated if inappropriate cleaning, disinfection or sterilisation procedures have been performed.
Primary packaging. The microbiological quality of primary packaging material is critical for sterile preparations. Vials, ampoules and stoppers shall be sterile and free of pyrogens before filling. For non-sterile preparations the microbiological quality of the packaging material is less critical. Because of the production process, bottles, tubes etc. will have only low levels of contamination, provided they have been packed, stored and handled under appropriate conditions.
Secondary contamination may occur during storage, transport, and administration of the product. The root cause for contamination during storage and transport is mainly insufficient closure integrity (see Sect. 24.3). Contamination during administration can be avoided by suitable design of the primary package (see Sect. 24.1) and appropriate instruction of the medical staff or patient (see Sect. 37.4). Tubes are less prone to contamination than jars. In hospitals eye drops should only be used for one specific patient and preferably for one specific eye.
19.5.3 Prevention of Microbial Contamination
Microbiological quality assurance and microbiological quality control should be part of the pharmaceutical quality system of any production site. Its main aim is to prevent microbial contamination in case of products required to be sterile (e.g. parenteral medicines), or to reduce the microbial counts and avoid objectionable micro-organisms in case of non-sterile products (e.g. tablets). The elements of a Pharmaceutical Quality System (PQS) that are crucial for microbiological quality regard adequate design of premises, procedures and controls. They are discussed in this section. The principles are included in current Good Manufacturing Practices (cGMP) guidelines (see Sect. 35.5.7), which are legally binding for pharmaceutical manufacturers. Microbiological quality assurance (QA) refers to the procedures in the quality system that ensures that microbiological requirements of the product are fulfilled. Microbiological quality control (QC) refers to the tests performed to verify that the product meets the required specifications.
19.5.3.1 Procedures
The following procedures and measures concerning facilities should mitigate the risk of microbiological contamination:
Qualified Personnel. Only trained and qualified personnel should enter areas where products are manufactured or prepared. Personnel should wear dedicated gowning which provides a physical barrier between the body and the working environment. The more critical the activity or product microbiological requirements, the stricter the gowning. Gowning may consist for instance of overalls, face masks, gloves (which are disinfected with ethanol) or even goggles in case of aseptic processing. Personnel that have apparent illnesses or infected wounds should be excluded from areas where open product is handled. Chapter Aseptic handling (Sect. 31.3.3) also discusses gowning.
Basic hygiene. Whereas the manufacturing of the most microbiological-critical products (e.g. parenteral, intravenous products) is strictly regulated and inspected, their preparation and application in hospitals is less subject to control. Nevertheless, measures to prevent microbial contamination or proliferation are equally, if not more important in this case. Basic hygiene rules include e.g. regular cleaning and if appropriate disinfecting the hands with alcohols, wearing sterile gloves for the preparation of parenterals, disinfecting the outer surface of critical products before opening. See Chap. 31 for detailed instructions on microbiological monitoring, disinfection procedures, operator qualification and validation of aseptic handling.
For basic hygiene at the preparation of non-sterile medicines the main points are given here.
Contact between the product and the operator is to be prevented. Thus:
Equipment and production processes shall be designed so that direct contact between operator and product is minimised.
Under no condition shall the product be touched with bare hands. If manipulation is unavoidable use utensils, such as forceps, or wear gloves. Gloves shall be changed when appropriate, particularly at every preparation and after obvious contamination such as sneezing and wiping the nose.
Refrain from talking above the product. Coughing and particularly sneezing are difficult to suppress. Wearing a facial mask and changing it at least every 2 h will considerably reduce the risk of contamination by this route. The operator shall inform his or her superior in case of a disease such as a cold.
Facial hair shall be appropriately covered; this may require the wearing of a head cover and a facial mask to cover moustaches and beards. This is also necessary from a safety point of view when operating with rotating equipment such as an ointment mill.
From a pure microbiological viewpoint wearing an overall doesn’t make sense other than the promotion of an attitude of working cleanly and neatly. Already after 1–2 h the overall bears as much contamination as the personal clothing. Directions for clothing are however also necessary to promote occupational safety and health (see Sect. 26.4.3). The overall has to remain in the preparation area (thus taken off at lunch and coffee breaks) and has to be cleaned according fixed schemes, such as daily and when visibly contaminated. The overall shall have long sleeves and cover completely personal clothing.
Washing hands must always occur:
At the start of preparation
If hands are visibly dirty
After using the toilets
After sneezing and wiping the nose
Between two different preparation processes, because of cross contamination
Nails have to be kept short and proper hand washing procedures include removal of watches, voluminous rings and bracelets (remaining off during the preparation process).
Washing hands technique requires preferably lukewarm water, soap from a dispenser, proper attention to thumbs, sufficient duration and proper drying with a towel because that will carry off micro-organisms too.
Controlled environments. Clean rooms in which pharmaceutical preparations are prepared processed, and packed are controlled for room pressure, humidity and temperature. They are segregated from other operating areas and may be entered via separate air locks for personnel and material. The incoming air of the clean rooms is filtered using HEPA (high efficiency particulate air) filters that may retain more than 99.995 % of 0.3 μm air particles (see Table 27.4). The higher the microbiological quality of the product or the critical the process step, the higher the clean room quality criteria. For instance in aseptic processing of sterile products for critical areas where the product is exposed to the surrounding environment, the air should not contain more than 3,520 particles of 0.5 μm size per cubic metre and should be devoid of viable micro-organisms (see air classifications in Tables 27.2 and 27.3). Clean room design should ensure that air, personnel and material flow is optimal to prevent microbial contamination from a less clean area to a cleaner area. Isolators have been introduced as an alternative to conventional clean rooms for aseptic production. These may be large installations, in which complex operations such as large-scale aseptic filling of syringes can be performed.
Controlled environments also regard to aseptic handling in pharmacies, where they are achieved using laminar flow cabinets, biological safety cabinets or isolators (see Sect. 28.4).
Cleaning and disinfection. The procedures for cleaning and disinfection (destruction of micro-organisms – but not necessarily spores – by chemical agents, see Sect. 31.4.3) of equipment parts that are in contact with the product have to be validated. In addition, for the more critical products that are required to be sterile, the equipment parts that are in contact with the product need to be sterilised. Sterilisation (destruction of micro-organisms including spores by heat) process of the manufacturing lines has also to be validated. For products, which are required to be sterile, the aseptic status of the production line is regularly evaluated by performing media fill simulations that consist of replacing the product with a microbial culture medium and evaluating if filled-media containers remain sterile.
Reducing bioburden. The preparation processes may reduce or even eliminate living micro-organisms. For instance on the preparation of tablets, the tableting of a granulate into a tablet may kill non-spore forming micro-organisms by the shearing forces of the interparticulate movement. Products required to be sterile are either sterile filtered (filter ≤0.2 μm pore diameter, see Sect. 30.6) or terminally sterilised directly in their container or package (e.g. steam sterilisation (Sect. 30.5.1), radiation (Sect. 30.5.3), ethylene oxide gas (30.5.4)).
Water distribution system. The distribution and storage systems for water that is used for cleaning, sterilisation and preparation should be devoid of biofilms. A distribution system may be controlled by continuously circulating heated water (>80 °C) in loops, avoiding one-way systems and dead ends, and application of disinfection steps such as adding ozone to the re-circulating water (see Sect. 27.5.2). The latter processes are often called sanitisation. In addition to the physico-chemical characteristics, water is monitored for microbiological counts. For preparation on a smaller scale, the storage of water should follow strict requirements as well, see further Sect. 23.3.1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


