Part 9: Terrorism and Clinical Medicine
261e | Microbial Bioterrorism |
Descriptions of the use of microbial pathogens as potential weapons of war or terrorism date from ancient times. Among the most frequently cited of such episodes are the poisoning of water supplies in the sixth century B.C. with the fungus Claviceps purpurea (rye ergot) by the Assyrians, the hurling of the dead bodies of plague victims over the walls of the city of Kaffa by the Tartar army in 1346, and the efforts by the British to spread smallpox to the Native American population loyal to the French via contaminated blankets in 1763. The tragic attacks on the World Trade Center and the Pentagon on September 11, 2001, followed closely by the mailing of letters containing anthrax spores to media and congressional offices through the U.S. Postal Service, dramatically changed the mindset of the American public regarding both our vulnerability to microbial bioterrorist attacks and the seriousness and intent of the federal government to protect its citizens against future attacks. Modern science has revealed methods of deliberately spreading or enhancing disease in ways not appreciated by our ancestors. The combination of basic research, good medical practice, and constant vigilance will be needed to defend against such attacks.
Although the potential impact of a bioterrorist attack could be enormous, leading to thousands of deaths and high morbidity rates, acts of bioterrorism would be expected to produce their greatest impact through the fear and terror they generate. In contrast to biowarfare, where the primary goal is destruction of the enemy through mass casualties, an important goal of bioterrorism is to destroy the morale of a society through fear and uncertainty. Although the actual biologic impact of a single act may be small, the degree of disruption created by the realization that such an attack is possible may be enormous. This was readily apparent with the impact on the U.S. Postal Service and the functional interruption of the activities of the legislative branch of the U.S. government following the anthrax attacks noted above. Thus, the key to the defense against these attacks is a highly functioning system of public health surveillance and education so that attacks can be quickly recognized and effectively contained. This is complemented by the availability of appropriate countermeasures in the form of diagnostics, therapeutics, and vaccines, both in response to and in anticipation of bioterrorist attacks.
The Working Group for Civilian Biodefense created a list of key features that characterize the elements of biologic agents that make them particularly effective as weapons (Table 261e-1). Included among these are the ease of spread and transmission of the agent and the presence of an adequate database to allow newcomers to the field to quickly apply the good science of others to bad intentions of their own. Agents of bioterrorism may be used in their naturally occurring forms, or they can be deliberately modified to deliver greater impact. Among the approaches to maximizing the deleterious effects of biologic agents are the genetic modification of microbes for the purposes of antimicrobial resistance or evasion by the immune system, creation of fine-particle aerosols, chemical treatment to stabilize and prolong infectivity, and alteration of host range through changes in surface proteins. Certain of these approaches fall under the category of weaponization, which is a term generally used to describe the processing of microbes or toxins in a manner that would ensure a devastating effect following release. For example, weaponization of anthrax by the Soviets involved the production of vast numbers of spores of appropriate size to reach the lower respiratory tract easily in a form that maintained aerosolization for prolonged periods of time and that could be delivered in a massive release, such as via widely dispersed bomblets.
KEY FEATURES OF BIOLOGIC AGENTS USED AS BIOWEAPONS |
Source: From L Borio et al: JAMA 287:2391, 2002; with permission.
The U.S. Centers for Disease Control and Prevention (CDC) classifies potential biologic threats into three categories: A, B, and C (Table 261e-2). Category A agents are the highest-priority pathogens. They pose the greatest risk to national security because they (1) can be easily disseminated or transmitted from person to person, (2) result in high mortality rates and have the potential for major public health impact, (3) might cause public panic and social disruption, and (4) require special action for public health preparedness. Category B agents are the second highest priority pathogens and include those that are moderately easy to disseminate, result in moderate morbidity rates and low mortality rates, and require specifically enhanced diagnostic capacity. Category C agents are the third highest priority. These include certain emerging pathogens to which the general population lacks immunity; that could be engineered for mass dissemination in the future because of availability, ease of production, and ease of dissemination; and that have a major public health impact and the potential for high morbidity and mortality rates. It should be pointed out, however, that these A, B, and C designations are empirical and, depending on evolving circumstances such as intelligence-based threat assessments, the priority rating of any given microbe or toxin could change. The CDC classification system also largely reflects the severity of illness produced by a given agent, rather than its accessibility to potential terrorists.
CDC CATEGORY A, B, AND C AGENTS |
Abbreviations: MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
Source: Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases.
CATEGORY A AGENTS
ANTHRAX
See also Chap. 175.
Bacillus anthracis as a Bioweapon Anthrax may be the prototypic disease of bioterrorism. Although rarely, if ever, spread from person to person, the illness embodies the other major features of a disease introduced through terrorism, as outlined in Table 261e-1. U.S. and British government scientists studied anthrax as a potential biologic weapon beginning approximately at the time of World War II (WWII). Offensive bioweapons activity including bioweapons research on microbes and toxins in the United States ceased in 1969 as a result of two executive orders by President Richard M. Nixon. Although the 1972 Biological and Toxin Weapons Convention Treaty outlawed research of this type worldwide, the Soviet Union produced and stored tons of anthrax spores for potential use as a bioweapon until at least the late 1980s. At present, there is suspicion that research on anthrax as an agent of bioterrorism is ongoing by several nations and extremist groups. One example of this was the release of anthrax spores by the Aum Shinrikyo cult in Tokyo in 1993. Fortunately, there were no casualties associated with that episode because of the inadvertent use of a nonpathogenic strain of anthrax by the terrorists.
The potential impact of anthrax spores as a bioweapon was clearly demonstrated in 1979 following the accidental release of spores into the atmosphere from a Soviet Union bioweapons facility in Sverdlovsk, Russia. Although total figures are not known, at least 77 cases of anthrax were diagnosed with certainty, of which 66 were fatal. These victims were exposed in an area within 4 km downwind of the facility, and deaths due to anthrax were also noted in livestock up to 50 km further downwind. Based on recorded wind patterns, the interval between the time of exposure and development of clinical illness ranged from 2 to 43 days. The majority of cases were within the first 2 weeks. Death typically occurred within 1–4 days following the onset of symptoms. It is likely that the widespread use of postexposure penicillin prophylaxis limited the total number of cases. The extended period of time between exposure and disease in some individuals supports the data from nonhuman primate studies, suggesting that anthrax spores can lie dormant in the respiratory tract for at least 4–6 weeks without evoking an immune response. This extended period of microbiologic latency following exposure poses a significant challenge for management of victims in the postexposure period.
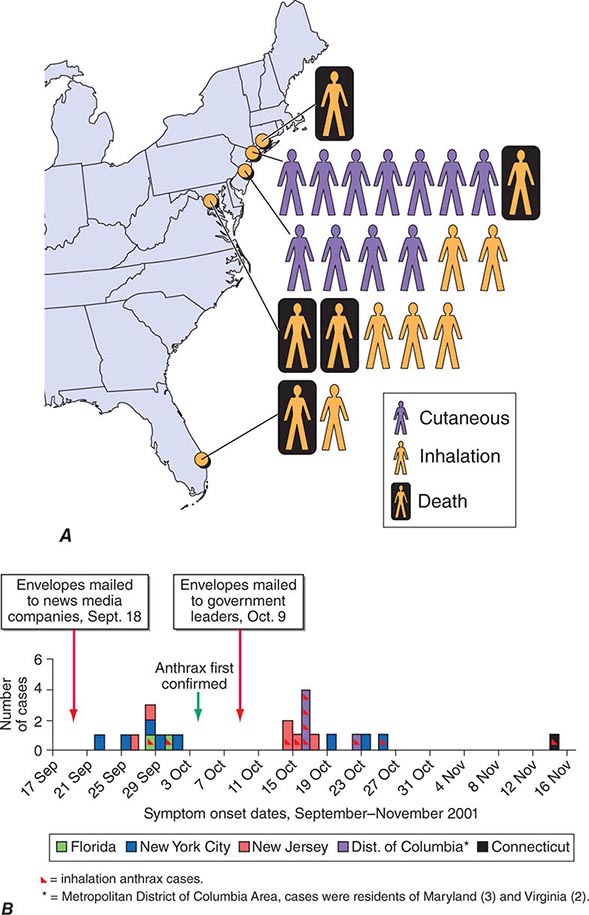
In September 2001, the American public was exposed to anthrax spores as a bioweapon delivered through the U.S. Postal Service by an employee of the United States Army Medical Research Institute for Infectious Diseases (USAMRIID) who had access to such materials and who committed suicide prior to being indicted for this crime. The CDC identified 22 confirmed or suspected cases of anthrax as a consequence of this attack. These included 11 patients with inhalational anthrax, of whom 5 died, and 11 patients with cutaneous anthrax (7 confirmed), all of whom survived (Fig. 261e-1). Cases occurred in individuals who opened contaminated letters as well as in postal workers involved in the processing of mail. A minimum of five letters mailed from Trenton, NJ, served as the vehicles for these attacks. One of these letters was reported to contain 2 g of material, equivalent to 100 billion to 1 trillion weapon-grade spores. Based on studies performed in the 1950s using monkeys exposed to aerosolized anthrax demonstrating that ~10,000 spores were required to produce lethal disease in 50% of animals exposed to this dose (the LD50), the contents of one letter had the theoretical potential, under optimal conditions, of causing illness or death in up to 50 million individuals. The strain used in this attack was the Ames strain. Although it was noted to have an inducible β-lactamase and to constitutively express a cephalosporinase, it was susceptible to all antibiotics standard for B. anthracis.
FIGURE 261e-1 Confirmed anthrax cases associated with bioterrorism: United States, 2001. A. Geographic location, clinical manifestation, and outcome of the 11 cases of confirmed inhalational and 11 cases of confirmed cutaneous anthrax. B. Epidemic curve for 22 cases of anthrax. (From DB Jernigan et al: Emerg Infect Dis 8:1019, 2002; with permission.)
Microbiology and Clinical Features Anthrax is caused by B. anthracis, a gram-positive, nonmotile, spore-forming rod that is found in soil and predominantly causes disease in herbivores such as cattle, goats, and sheep. Anthrax spores can remain viable for decades. The remarkable stability of these spores makes them an ideal bioweapon, and their destruction in decontamination activities can be a challenge. Naturally occurring human infection is generally the result of contact with anthrax-infected animals or animal products such as goat hair in textile mills or animal skins used in making drums. While an LD50 of 10,000 spores is a generally accepted number, it has also been suggested that as few as one to three spores may be adequate to cause disease in some settings. Advanced technology is likely to be necessary to generate a bioweapon containing spores of the optimal size (1–5 μm) to travel to the alveolar spaces.
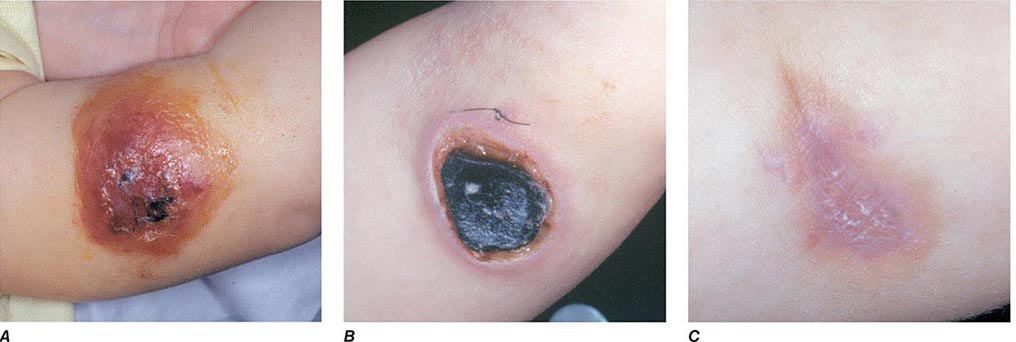
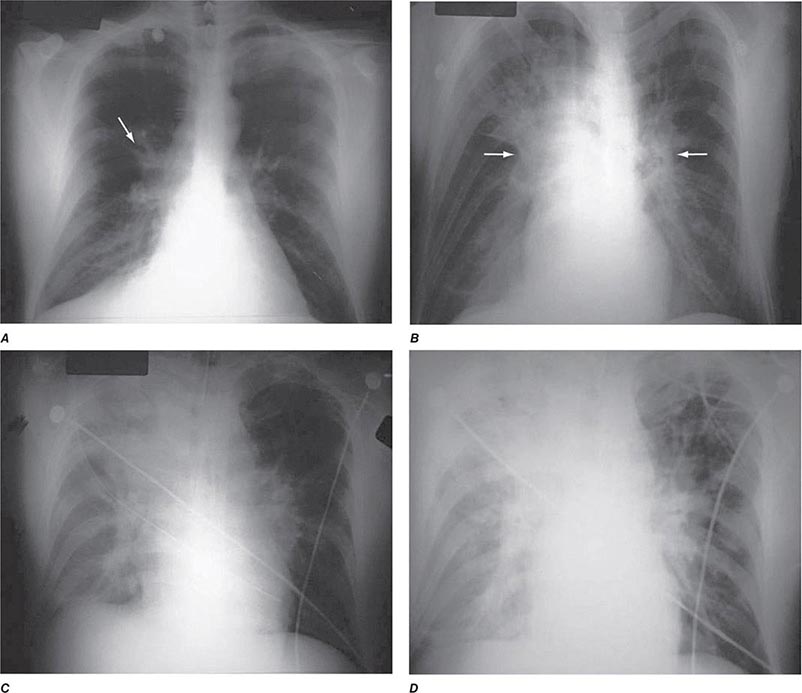
The three major clinical forms of anthrax are gastrointestinal, cutaneous, and inhalational. Gastrointestinal anthrax typically results from the ingestion of contaminated meat; the condition is rarely seen and is unlikely to be the result of a bioterrorism event. The lesion of cutaneous anthrax typically begins as a papule following the introduction of spores through an opening in the skin. This papule then evolves to a painless vesicle followed by the development of a coal-black, necrotic eschar (Fig. 261e-2). It is the Greek word for coal (anthrax) that gives the organism and the disease its name. Cutaneous anthrax was ~20% fatal prior to the availability of antibiotics. Inhalational anthrax is the form most likely to be responsible for death in the setting of a bioterrorist attack. It occurs following the inhalation of spores that become deposited in the alveolar spaces. These spores are phagocytized by macrophages and transported to the mediastinal and peribronchial lymph nodes where they germinate, leading to active bacterial growth and elaboration of the bacterial products edema toxin and lethal toxin. Subsequent hematogenous spread of bacteria is accompanied by cardiovascular collapse and death. The earliest symptoms are typically a viral-like prodrome with fever, malaise, and abdominal and/or chest symptoms that progress over the course of a few days to a moribund state. Characteristic findings on chest x-ray include mediastinal widening and pleural effusions (Fig. 261e-3). Although initially thought to be 100% fatal, the experiences at Sverdlovsk in 1979 and in the United States in 2001 (see below) indicate that with prompt initiation of antibiotic therapy, survival is possible. The characteristics of the 11 cases of inhalational anthrax diagnosed in the United States in 2001 following exposure to contaminated letters postmarked September 18 or October 9, 2001, followed the classic pattern established for this illness, with patients presenting with a rapidly progressive course characterized by fever, fatigue or malaise, nausea or vomiting, cough, and shortness of breath. At presentation, the total white blood cell counts were ~10,000 cells/μL; transaminases tended to be elevated, and all 11 had abnormal findings on chest x-ray and computed tomography (CT). Radiologic findings included infiltrates, mediastinal widening, and hemorrhagic pleural effusions. For cases in which the dates of exposure were known, symptoms appeared within 4–6 days. Death occurred within 7 days of diagnosis in the five fatal cases (overall mortality rate 55%). Rapid diagnosis and prompt initiation of antibiotic therapy were key to survival.
FIGURE 261e-2 Clinical manifestations of a pediatric case of cutaneous anthrax associated with the bioterrorism attack of 2001. The lesion progresses from vesicular on day 5 (A) to necrotic with the classic black eschar on day 12 (B) to a healed scar 2 months later (C). (Photographs provided by Dr. Mary Wu Chang. Part A reproduced with permission from KJ Roche et al: N Engl J Med 345:1611, 2001 and Parts B and C reproduced with permission from A Freedman et al: JAMA 287:869, 2002.)
FIGURE 261e-3 Progression of chest x-ray findings in a patient with inhalational anthrax. Findings evolved from subtle hilar prominence and right perihilar infiltrate to a progressively widened mediastinum, marked perihilar infiltrates, peribronchial cuffing, and air bronchograms. (From L Borio et al: JAMA 286:2554, 2001; with permission.)
CLINICAL SYNDROMES, PREVENTION, AND TREATMENT STRATEGIES FOR DISEASES CAUSED BY CATEGORY A AGENTS |
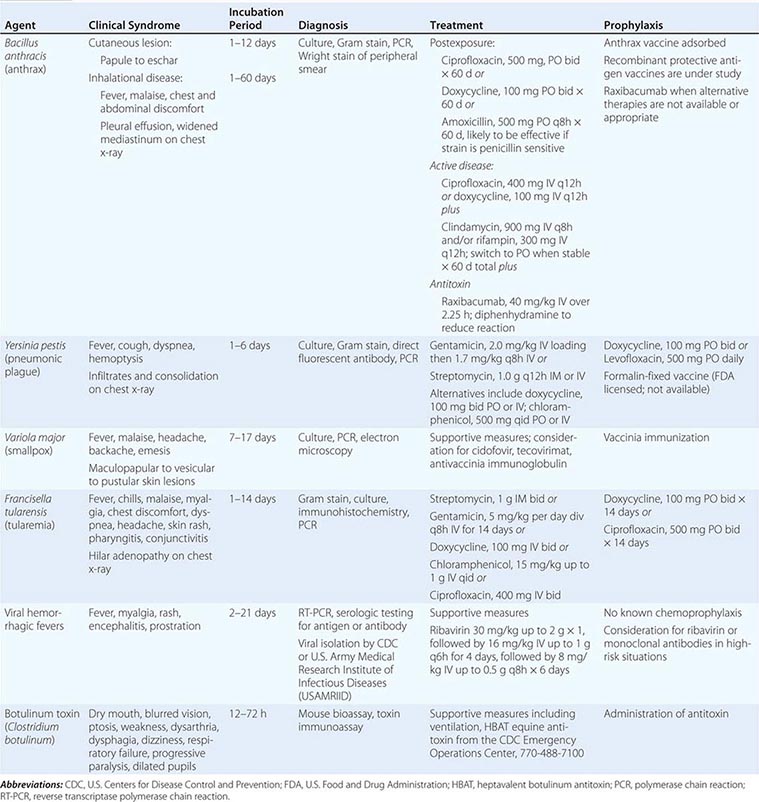
Vaccination and Prevention The first successful vaccine for anthrax was developed for animals by Louis Pasteur in 1881. At present, the single vaccine licensed for human use is a product produced from the cell-free culture supernatant of an attenuated, nonencapsulated strain of B. anthracis (Stern strain), referred to as anthrax vaccine adsorbed (AVA). Clinical trials for safety in humans and efficacy in animals are currently under way to evaluate the role of recombinant protective antigen as an alternative to AVA. In a postexposure setting in nonhuman primates, a 2-week course of AVA plus ciprofloxacin was found to be superior to ciprofloxacin alone in preventing the development of clinical disease and death. Although the current recommendation for postexposure prophylaxis is 60 days of antibiotics, it would seem prudent to include immunization with anthrax vaccine if available. Given the potential for B. anthracis to be engineered to express penicillin resistance, the empirical regimen of choice in this setting is either ciprofloxacin or doxycycline. In settings where these approaches are not available or appropriate, one can administer the antitoxin monoclonal antibody raxibacumab.
PLAGUE
See also Chap. 196.
Yersinia pestis as a Bioweapon Although it lacks the environmental stability of anthrax, the highly contagious nature and high mortality rate of plague make it a close to ideal agent of bioterrorism, particularly if delivered in a weaponized form. Occupying a unique place in history, plague has been alleged to have been used as a biologic weapon for centuries. The catapulting of plague-infected corpses into besieged fortresses is a practice that was first noted in 1346 during the assault of the Crimean city of Kaffa by the Mongolian Tartars. Although unlikely to have resulted in disease transmission, some believe that this event may have played a role in the start of the Black Death pandemic of the fourteenth and fifteenth centuries in Europe. Given that plague was already moving across Asia toward Europe at this time, it is unclear whether such an allegation is accurate. During WWII, the infamous Unit 731 of the Japanese army was reported to have repeatedly dropped plague-infested fleas over parts of China, including Manchuria. These drops were associated with subsequent outbreaks of plague in the targeted areas. Following WWII, the United States and the Soviet Union conducted programs of research on how to create aerosolized Y. pestis that could be used as a bioweapon to cause primary pneumonic plague. As mentioned above, plague was thought to be an excellent bioweapon due to the fact that in addition to causing infection in those inhaling the aerosol, significant numbers of secondary cases of primary pneumonic plague would also likely occur due to the contagious nature of the disease and person-to-person transmission via respiratory aerosol. Secondary reports of research conducted during that time suggest that organisms remain viable for up to 1 h and can be dispersed for distances up to 10 km. Although the offensive bioweapons program in the United States was terminated prior to production of sufficient quantities of plague organisms for use as a weapon, it is believed that Soviet scientists did manufacture quantities sufficient for such a purpose. It has also been reported that more than 10 Soviet institutes and >1000 scientists were working with plague as a biologic weapon. Of concern is the fact that in 1995 a microbiologist in Ohio was arrested for having obtained Y. pestis in the mail from the American Type Culture Collection, using a credit card and a false letterhead. In the wake of this incident, the U.S. Congress passed a law in 1997 requiring that anyone intending to send or receive any of 42 different agents that could potentially be used as bioweapons first register with the CDC.
Microbiology and Clinical Features Plague is caused by Y. pestis, a nonmotile, gram-negative bacillus that exhibits bipolar, or “safety pin,” staining with Wright, Giemsa, or Wayson stains. It has had a major impact on the course of history, thus adding to the element of fear evoked by its mention. The earliest reported plague epidemic was in 224 B.C. in China. The most infamous pandemic began in Europe in the fourteenth century, during which time one-third to one-half of the entire population of Europe was killed. During a plague outbreak in India in 1994, even though the number of confirmed cases was relatively small, it is estimated that 500,000 individuals fled their homes in fear of this disease. In the first decade of the twenty-first century, 21,725 cases of plague were reported to the World Health Organization (WHO). Over 90% of these cases were from Africa, and the overall case fatality rate was 7.4%.
The clinical syndromes of plague generally reflect the mode of infection. Bubonic plague is the consequence of an insect bite; primary pneumonic plague arises through the inhalation of bacteria. Most of the plague seen in the world today is bubonic plague and is the result of a bite by a plague-infected flea. In part as a consequence of past pandemics, plague infection of rodents exists widely in nature, including in the southwestern United States, and each year thousands of cases of plague occur worldwide through contact with infected animals or fleas. Following inoculation of regurgitated bacteria into the skin by a flea bite, organisms travel through the lymphatics to regional lymph nodes, where they are phagocytized but not destroyed. Inside the cell, they multiply rapidly leading to inflammation, painful lymphadenopathy with necrosis, fever, bacteremia, septicemia, and death. The characteristic enlarged, inflamed lymph nodes, or buboes, give this form of plague its name. In some instances, patients may develop bacteremia without lymphadenopathy following infection, a condition referred to as primary septicemic plague. Extensive ecchymoses may develop due to disseminated intravascular coagulation, and gangrene of the digits and/or nose may develop in patients with advanced septicemic plague. It is thought that this appearance of some patients gave rise to the term Black Death in reference to the plague epidemic of the fourteenth and fifteenth centuries. Some patients may develop pneumonia (secondary pneumonic plague) as a complication of bubonic or septicemic plague. These patients may then transmit the agent to others via the respiratory route, causing cases of primary pneumonic plague. Primary pneumonic plague is the manifestation most likely to occur as the result of a bioterrorist attack, with an aerosol of bacteria spread over a wide area or a particular environment that is densely populated. In this setting, patients would be expected to develop fever, cough with hemoptysis, dyspnea, and gastrointestinal symptoms 1–6 days following exposure. Clinical features of pneumonia would be accompanied by pulmonary infiltrates and consolidation on chest x-ray. In the absence of antibiotics, the mortality rate of this form of plague is on the order of 85%, and death usually occurs within 2–6 days.
Vaccination and Prevention A formalin-fixed, whole-organism vaccine was licensed by the FDA for the prevention of plague. That vaccine is no longer being manufactured, but its potential value as a current countermeasure against bioterrorism would likely have been modest at best as it was ineffective against animal models of primary pneumonic plague. Efforts are under way to develop a second generation of vaccines that will protect against aerosol challenge. Among the candidates being tested are recombinant forms of the fraction 1 capsular (F1) antigen and the virulence component of the type III secretion apparatus (V) antigen of Y. pestis. It is likely that doxycycline or levofloxacin would provide coverage in a chemoprophylaxis setting. Unlike the case with anthrax, in which one has to be concerned about the persistence of ungerminated spores in the respiratory tract, the duration of prophylaxis against plague need only extend to 7 days following exposure.
SMALLPOX
See also Chap. 220e.
Variola Virus as a Bioweapon Given that most of the world’s population was once vaccinated against smallpox, variola virus would not have been considered a good candidate as a bioweapon 30 years ago. However, with the cessation of immunization programs in the United States in 1972 and throughout the world in 1980 due to the successful global eradication of smallpox, close to 50% of the U.S. population is fully susceptible to smallpox today. Given its infectious nature and the 10–30% mortality rate in unimmunized individuals, the deliberate spread of this virus could have a devastating effect on our society and unleash a previously conquered deadly disease. It is estimated that an initial infection of 50–100 persons in a first generation of cases could expand by a factor of 10–20 with each succeeding generation in the absence of any effective containment measures. Although the likely implementation of an effective public health response makes this scenario unlikely, it does illustrate the potential damage and disruption that can result from a smallpox outbreak.
In 1980, the WHO recommended that all immunization programs be terminated; that representative samples of variola virus be transferred to two locations, one at the CDC in Atlanta, GA, in the United States and the other at the Institute of Virus Preparations in the Soviet Union; and that all other stocks of smallpox be destroyed. Several years later, it was recommended that these two authorized collections be destroyed. However, these latter recommendations were placed on hold in the wake of increased concerns on the use of variola virus as a biologic weapon and thus the need to maintain an active program of defensive research. Many of these concerns were based on allegations made by former Soviet officials that extensive programs had been in place in that country for the production and weaponization of large quantities of smallpox virus. The dismantling of these programs with the fall of the Soviet Union and the subsequent weakening of security measures led to fears that stocks of Variola major may have made their way to other countries or terrorist organizations. In addition, accounts that efforts had been taken to produce recombinant strains of Variola that would be more virulent and more contagious than the wild-type virus have led to an increase in the need to be vigilant for the reemergence of this often fatal infectious disease.
Microbiology and Clinical Features Smallpox is caused by one of two variants of variola virus, V. major and V. minor. Variola is a double-strand DNA virus and member of the Orthopoxvirus genus of the Poxviridae family. Infections with V. minor are generally less severe than those of V. major, with milder constitutional symptoms and lower mortality rates; thus V. major is the only one considered to be a viable bioweapon. Infection with V. major typically occurs following contact with an infected person. Patients are infectious from the time that a maculopapular rash appears on the skin and oropharynx through the resolution and scabbing of the pustular lesions. Infection occurs principally during close contact, through the inhalation of saliva droplets containing virus from the oropharyngeal exanthem. Aerosolized material from contaminated clothing or linen can also spread infection. Several days after exposure, a primary viremia is believed to occur that results in dissemination of virus to lymphoid tissues. A secondary viremia occurs ~4 days later that leads to localization of infection in the dermis. Approximately 12–14 days following the initial exposure, the patient develops high fever, malaise, vomiting, headache, backache, and a maculopapular rash that begins on the face and extremities and spreads to the trunk (centripetal) with lesions in the same developmental stage in any given location. This is in contrast to the rash of varicella (chickenpox) that begins on the trunk and face and spreads to the extremities (centrifugal) with lesions at all stages of development. The lesions are initially maculopapular and evolve to vesicles that eventually become pustules and then scabs. The oral mucosa also develops maculopapular lesions that evolve to ulcers. The lesions appear over a period of 1–2 days and evolve at the same rate. Although virus can be isolated from the scabs on the skin, the conventional thinking is that once the scabs have formed the patient is no longer contagious. Smallpox is associated with 10–30% mortality rates, with patients typically dying of severe systemic illness during the second week of symptoms. Historically, ~5–10% of naturally occurring smallpox cases take either of two highly virulent atypical forms, classified as hemorrhagic and malignant. These are difficult to diagnose because of their atypical presentations. The hemorrhagic form is uniformly fatal and begins with the relatively abrupt onset of a severely prostrating illness characterized by high fevers and severe headache and back and abdominal pain. This form of the illness resembles a severe systemic inflammatory syndrome, in which patients have a high viremia but die without developing the characteristic rash. Cutaneous erythema develops accompanied by petechiae and hemorrhages into the skin and mucous membranes. Death usually occurs within 5–6 days. The malignant, or “flat,” form of smallpox is frequently fatal and has an onset similar to the hemorrhagic form, but with confluent skin lesions developing more slowly and never progressing to the pustular stage.
Vaccination and Prevention In 1796, Edward Jenner demonstrated that deliberate infection with cowpox virus could prevent illness on subsequent exposure to smallpox. Today, smallpox is a preventable disease following immunization with vaccinia. The current dilemma facing our society regarding assessment of the risk and benefit of smallpox vaccination is that the degree of risk that someone will deliberately and effectively release smallpox into our society is unknown. Given that there are well-described risks associated with vaccination, the degree of risk/benefit for the general population does not favor immunization. As a prudent first step in preparedness for a smallpox attack, however, members of the U.S. armed services received primary or booster immunizations with vaccinia before 1990 and after 2002. In addition, a number of civilian health care workers who comprise smallpox-response teams at the state and local public health level have been vaccinated.
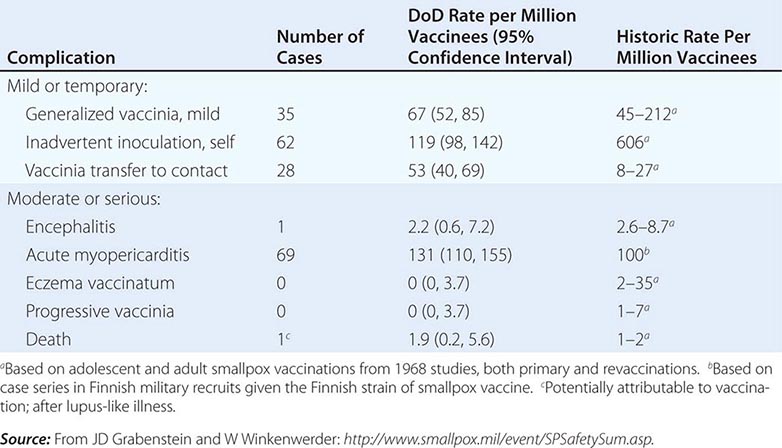
Initial fears regarding the immunization of a segment of the American population with vaccinia when there are more individuals receiving immunosuppressive drugs and other immunocompromised patients than ever before were dispelled by the data generated from the military and civilian immunization campaigns of 2002–2004. Adverse event rates for the first 450,000 immunizations were similar to and, in certain categories of adverse events, even lower than those from prior historic data, in which most severe sequelae of vaccination occurred in young infants (Table 261e-4). In addition, 11 patients with early-stage HIV infection were inadvertently immunized without problem. One significant concern during that immunization campaign, however, was the description of a syndrome of myopericarditis, which had not been appreciated during prior immunization campaigns with vaccinia. In an effort to provide a safer vaccine to protect against smallpox, ACAM 2000, a cloned virus propagated in tissue culture, was developed and became the first second-generation smallpox vaccine to be licensed. This vaccine is now the only vaccinia product currently licensed in the United States and has been used by the U.S. military since 2008. It is part of the U.S. government stockpile. Research continues on attenuated forms of vaccinia such as modified vaccinia Ankara (MVA). Vaccinia immune globulin is available to treat those who experience a severe reaction to immunization with vaccinia.
COMPLICATIONS FROM 438,134 ADMINISTRATIONS OF VACCINIA DURING THE U.S. DEPARTMENT OF DEFENSE (DOD) SMALLPOX IMMUNIZATION CAMPAIGN INITIATED IN DECEMBER 2002 |
TULAREMIA
See also Chap. 195.
Francisella tularensis as a Bioweapon Tularemia has been studied as an agent of bioterrorism since the mid-twentieth century. It has been speculated by some that the outbreak of tularemia among German and Soviet soldiers during fighting on the Eastern Front during WWII was the consequence of a deliberate release. Unit 731 of the Japanese army studied the use of tularemia as a bioweapon during WWII. Large preparations were made for mass production of F. tularensis by the United States, but no stockpiling of any agent took place. Stocks of F. tularensis were reportedly generated by the Soviet Union in the mid-1950s. It has also been suggested that the Soviet program extended into the era of molecular biology and that some strains were engineered to be resistant to common antibiotics. F. tularensis is an extremely infectious organism, and human infections have occurred from merely examining an uncovered petri dish streaked with colonies. Given these facts, it is reasonable to conclude that this organism might be used as a bioweapon through either an aerosol or contamination of food or drinking water.
Microbiology and Clinical Features Although similar in many ways to anthrax and plague, tularemia, also referred to as rabbit fever or deer fly fever, is neither as lethal nor as fulminant as either of these other two category A bacterial infections. It is, however, extremely infectious, and as few as 10 organisms can lead to establishment of infection. Despite this fact, it is not spread from person to person. Tularemia is caused by F. tularensis, a small, nonmotile, gram-negative coccobacillus. Although it is not a spore-forming organism, it is a hardy bacterium that can survive for weeks in the environment. Infection typically comes from insect bites or contact with organisms in the environment. Infections have occurred in laboratory workers studying the agent. Large waterborne outbreaks have been recorded. It is most likely that the outbreak among German and Russian soldiers and Russian civilians noted above during WWII represented a large waterborne tularemia outbreak in a Tularensis-enzootic area devastated by warfare.
Humans can become infected through a variety of environmental sources. Infection is most common in rural areas where a variety of small mammals may serve as reservoirs. Human infections in the summer are often the result of insect bites from ticks, flies, or mosquitoes that have bitten infected animals. In colder months, infections are most likely the result of direct contact with infected mammals and are most common in hunters. In these settings, infection typically presents as a systemic illness with an area of inflammation and necrosis at the site of tissue entry. Drinking of contaminated water may lead to an oropharyngeal form of tularemia characterized by pharyngitis with cervical and/or retropharyngeal lymphadenopathy (Chap. 195). The most likely mode of dissemination of tularemia as a biologic weapon would be as an aerosol, as has occurred in a number of natural outbreaks in rural areas, including Martha’s Vineyard in the United States. Approximately 1–14 days following exposure by this route, one would expect to see inflammation of the airways with pharyngitis, pleuritis, and bronchopneumonia. Typical symptoms would include the abrupt onset of fever, fatigue, chills, headache, and malaise (Table 261e-3). Some patients might experience conjunctivitis with ulceration, pharyngitis, and/or cutaneous exanthems. A pulse-temperature dissociation might be present. Approximately 50% of patients would show a pulmonary infiltrate on chest x-ray. Hilar adenopathy might also be present, and a small percentage of patients could have adenopathy without infiltrates. The highly variable presentation makes acute recognition of aerosol-disseminated tularemia very difficult. The diagnosis would likely be made by immunohistochemistry, molecular techniques, or culture of infected tissues or blood. Untreated, mortality rates range from 5 to 15% for cutaneous routes of infection and from 30 to 60% for infection by inhalation. Since the advent of antibiotic therapy, these rates have dropped to <2%.
Vaccination and Prevention There are no vaccines currently licensed for the prevention of tularemia. Although a live, attenuated strain of the organism has been used in the past with some reported success, there are inadequate data to support its widespread use at this time. Development of a vaccine for this agent is an important part of the current biodefense research agenda. In the absence of an effective vaccine, postexposure chemoprophylaxis with either doxycycline or ciprofloxacin appears to be a reasonable approach (Table 261e-3).
VIRAL HEMORRHAGIC FEVERS
See also Chaps. 233 and 234.
Hemorrhagic Fever Viruses as Bioweapons Several of the hemorrhagic fever viruses have been reported to have been weaponized by the Soviet Union and the United States. Nonhuman primate studies indicate that infection can be established with very few virions and that infectious aerosol preparations can be produced. Under the guise of wanting to aid victims of an Ebola outbreak, members of the Aum Shinrikyo cult in Japan were reported to have traveled to central Africa in 1992 in an attempt to obtain Ebola virus for use in a bioterrorist attack. Thus, although there has been no evidence that these agents have ever been used in a biologic attack, there is clear interest in their potential for this purpose.
Microbiology and Clinical Features The viral hemorrhagic fevers are a group of illnesses caused by any one of a number of similar viruses (Table 261e-2). These viruses are all enveloped, single-strand RNA viruses that are thought to depend on a host reservoir for long-term survival. Although rodents or insects have been identified as the hosts for some of these viruses, for others the hosts are unknown. These viruses tend to be geographically restricted according to the migration patterns of their hosts. Great apes are not a natural reservoir for Ebola virus, but large numbers of these animals in sub-Saharan Africa have died from Ebola infection over the past decade. Humans can become infected with hemorrhagic fever viruses if they come into contact with an infected host or other infected animals. Person-to-person transmission, largely through direct contact with virus-containing body fluids, has been documented for Ebola, Marburg, and Lassa viruses and rarely for the New World arenaviruses. Although there is no clear evidence of respiratory spread among humans, these viruses have been shown in animal models to be highly infectious by the aerosol route. This, coupled with mortality rates as high as 90%, makes them excellent candidate agents of bioterrorism.
The clinical features of the viral hemorrhagic fevers vary depending on the particular agent (Table 261e-3). Initial signs and symptoms typically include fever, myalgia, prostration, and disseminated intravascular coagulation with thrombocytopenia and capillary hemorrhage. These findings are consistent with a cytokine-mediated systemic inflammatory syndrome. A variety of different maculopapular or erythematous rashes may be seen. Leukopenia, temperature-pulse dissociation, renal failure, and seizures may also be part of the clinical presentation.
Outbreaks of most of these diseases are sporadic and unpredictable. As a consequence, most studies of pathogenesis have been performed using laboratory animals. The diagnosis should be suspected in anyone with temperature >38.3°C for <3 weeks who also exhibits at least two of the following: hemorrhagic or purpuric rash, epistaxis, hematemesis, hemoptysis, or hematochezia in the absence of any other identifiable cause. In this setting, samples of blood should be sent after consultation to the CDC or the USAMRIID for serologic testing for antigen and antibody as well as reverse transcriptase polymerase chain reaction (RT-PCR) testing for hemorrhagic fever viruses. All samples should be handled with double-bagging. Given how little is known regarding the human-to-human transmission of these viruses, appropriate isolation measures would include full barrier precautions with negative-pressure rooms and use of powered air-purifying respirators (PAPRs). Unprotected skin contact with cadavers has been implicated in the transmission of certain hemorrhagic fever viruses such as Ebola, so it is recommended that autopsies of suspected cases be performed using the strictest measures for protection and that burial or cremation be performed promptly without embalming.