, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Membranous nephropathy is a major cause of the nephrotic syndrome in adults [1, 2]. Only in the past decades has it been surpassed by focal and segmental glomerulosclerosis as the main cause of the nephrotic syndrome in this age group [3, 4]. Membranous nephropathy develops mostly as an idiopathic disorder but can also be seen secondary to hepatitis B and other virus infections; Sjögren’s syndrome; transplantation; systemic lupus erythematosus; syphilis; exposure to certain drugs and heavy metals (e.g., penicillamine, bucillamine, gold, mercuric chloride); malignancies including carcinomas, carcinoids, sarcomas, lymphomas, and leukemias; and other systemic conditions [1, 5–7]. Idiopathic membranous nephropathy, which we now know to have an autoimmune origin, must be distinguished from membranous lupus glomerulonephritis [8], as discussed in Chap. 8. Synonyms for membranous nephropathy are membranous glomerulonephritis and membranous glomerulopathy.
Introduction/Clinical Setting
Membranous nephropathy is a major cause of the nephrotic syndrome in adults [1, 2]. Only in the past decades has it been surpassed by focal and segmental glomerulosclerosis as the main cause of the nephrotic syndrome in this age group [3, 4]. Membranous nephropathy develops mostly as an idiopathic disorder but can also be seen secondary to hepatitis B and other virus infections; Sjögren’s syndrome; transplantation; systemic lupus erythematosus; syphilis; exposure to certain drugs and heavy metals (e.g., penicillamine, bucillamine, gold, mercuric chloride); malignancies including carcinomas, carcinoids, sarcomas, lymphomas, and leukemias; and other systemic conditions [1, 5–7]. Idiopathic membranous nephropathy, which we now know to have an autoimmune origin, must be distinguished from membranous lupus glomerulonephritis [8], as discussed in Chap. 8. Synonyms for membranous nephropathy are membranous glomerulonephritis and membranous glomerulopathy.
Idiopathic membranous nephropathy occurs mostly in adults with a peak incidence in the fourth and fifth decades; at all ages men are more often affected than women. Patients present most often with a nephrotic syndrome (approximately 80 %), with an onset that is often more gradual than occurs with minimal change disease [6]. Microscopic hematuria is a common concurrent manifestation. Blood pressure and renal function are often normal at the time of presentation. The clinical course is often waxing and waning, as indicated by the level of proteinuria, and spontaneous remissions and/or remissions induced by therapy are common. Some patients present with only asymptomatic proteinuria or hematuria. In general the prognosis is excellent in children, whereas in adults approximately 30–40 % of patients progress to chronic renal failure with depression of the glomerular filtration rate (GFR) [6, 9]. In secondary membranous nephropathy the underlying disease determines the prognosis. The therapeutic approach to patients with membranous nephropathy remains suboptimal [10–13].
Pathologic Findings
Light Microscopy
Morphologic changes in membranous nephropathy are usually present in all glomeruli found in a biopsy, with little variation in the severity of the lesions between glomeruli. Morphologic lesions, however, can differ between patients or between biopsies taken from one patient at different time points. This is caused by the evolutionary pathologic changes occurring in the glomerular capillary walls over time. The histologic changes can be very subtle and sometimes are hardly or not at all visible, thereby illustrating the need for performing immunofluorescence and electron microscopic studies to establish the diagnosis.
In most cases the glomerular capillary wall is diffusely thickened as visualized by different histologic stains, as a result of the presence of nonargyrophilic, subepithelially localized immune deposits. In early stages a silver methenamine stain may reveal basement membranes completely normal in histologic appearance and thickness. Irregular thickenings of the glomerular basement membrane may grow around the immune deposits and appear in the silver-stained histologic sections as black “spikes” which project outwards to urinary space (Fig. 2.1). These are at first small and segmental, but they often grow in the course of the disease and increasingly serve to separate the deposits and incorporate them into basement membranes. Three dimensionally the spikes create spaces in the capillary walls that can be likened to “craters,” which appear as lucencies or rarefactions at sites where the capillary walls are cut tangentially. Spike formation results from the presence of subepithelial deposits, which trigger the overlying epithelial cells to increase their production of extracellular matrix, especially laminin [14]. The glomeruli in idiopathic membranous nephropathy typically lack inflammatory cell infiltration. Mesangial alterations such as proliferation are uncommon and should prompt a search for secondary causes of membranous nephropathy. Concurrent focal and segmental glomerulosclerosis, when present, imparts a worse prognosis [15, 16]. Segmental necrosis or crescent formation is not characteristic of this disorder and should prompt consideration of a concurrent disease process such as lupus nephritis, ANCA-associated glomerulonephritis, or superimposed anti-glomerular basement membrane antibody-mediated disease [17, 18].
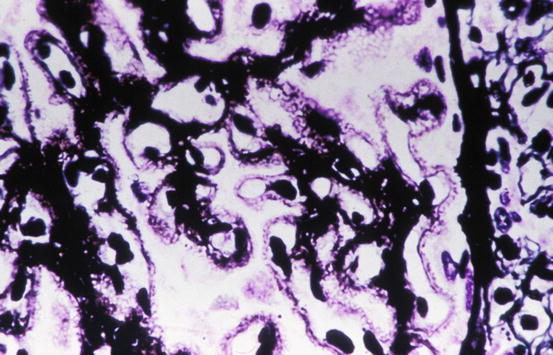

Fig. 2.1
Glomerulus showing thickening of glomerular basement membrane (GBM) and subepithelial “spikes” in membranous nephropathy (silver methenamine stain)
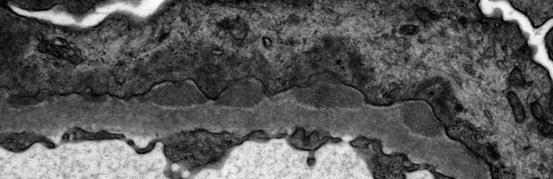
In membranous nephropathy, there is a protein leak into the urinary space consequent to the structural alternations to the glomerular filtration barrier, and the tubules downstream of the glomeruli often contain protein reabsorption droplets. Foam cells may be present in the interstitium or between tubular epithelial cells and may be related to the hyperlipidemia and reabsorption of filtered lipoproteins, as can be seen also in nephrotic syndrome due to other causes. With progression of the glomerular lesions, nephron loss and interstitial fibrosis may occur. In late stages of cases of membranous nephropathy that have progressed to chronic kidney disease, a proportion of the glomeruli show nonspecific global sclerosis. Interstitial blood vessels are mostly without specific abnormalities in membranous nephropathy, though they may show changes such as arteriosclerosis that may be a consequence of concurrent injury processes such as hypertension on aging. The extent of interstitial damage correlates strongly with prognosis [19].
Immunofluorescence Microscopy
Immunofluorescence investigations in membranous nephropathy reveal granular deposits of immune reactants, typically with a uniform distribution and pattern in all of the glomeruli present in a specific biopsy, which follow the contours of the glomerular basement membranes. Immunoelectron microscopic studies have shown that these immune reactants are present in the electron-dense deposits described in Electron Microscopy below. The deposits can sometimes be very finely granular and extensively distributed, which may lead to the immunofluorescence pattern being falsely interpreted as linear. In a minority of cases, the deposits may be sparse or only segmentally distributed. Deposits in the mesangium are absent in most cases. The most commonly identified component in the immune deposits is immunoglobulin G (IgG) (Fig. 2.2), and in cases of idiopathic membranous nephropathy, the IgG is predominantly of subclass IgG4 [5, 20]. In addition, C3 is often present, while concurrent deposits of IgM and IgA are found in some cases. When IgG, IgA, IgM, C3, and C1q are all found (“full house”), suspicion of lupus membranous nephritis should arise [8].
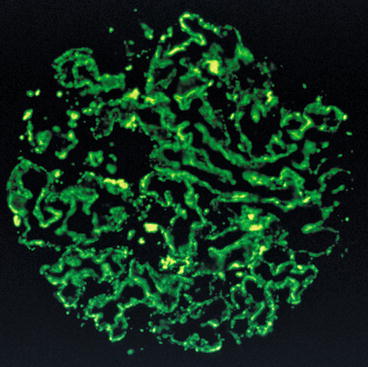

Fig. 2.2
Immunofluorescence showing diffuse fine granular distribution pattern of immunoglobulin G (IgG) along the glomerular basement membranes in membranous nephropathy
Electron Microscopy
The presence of subepithelial electron-dense deposits, whether widespread or sparsely distributed in the glomerular capillary walls, is the characteristic finding in membranous nephropathy (Figs. 2.3 and 2.4). The electron-dense deposits typically have a finely granular appearance without identifiable substructure. Electron microscopy also commonly demonstrates the basement membrane changes and “spikes” identified in silver-stained histologic preparations (Fig. 2.4). These changes contribute to progressive incorporation of the electron-dense immune deposits into the capillary wall, where the deposits may then occupy an intramenbranous location. Over time, the deposits can become less electron dense and even become electron lucent, presumably due to degradative processes that most likely are some form of proteolytic digestion. The spikes can be found in different stages of development, between and around the electron-dense deposits (Fig. 2.4). The subepithelial deposits are in contact with the glomerular epithelial cells, at least initially. In these cells the cytoplasm close to the deposits often shows condensation of intracytoplasmic actin filaments, and there is extensive effacement of the foot processes overlying the capillary walls (Figs. 2.3 and 2.4). The epithelial cells may also show reabsorption droplets and microvillus transformation as a nonspecific indication of cell injury. The changes in the distribution of deposits in the glomerular capillary wall, the progressive electron lucency of the deposits, and the thickening of the basement membranes as spikes of new basement membrane matrix are formed and as deposits are incorporated into the capillary wall are indicative of a sequence of events corresponding to early to late changes in the evolution of membranous nephropathy. This progressive evolution has been described in the classification of membranous nephropathy by Ehrenreich and Churg and has been generally divided into four stages of progression [21]. In stage I light microscopic changes are absent, but immunofluorescence shows granular aggregates of immunoglobulins, and electron microscopy shows subepithelial deposits without prominent basement membrane alterations (Fig. 2.3). In stage II spikes are present and the basement membrane matrices that progressively surround the immune deposits are visualized by electron microscopy. This may progress to stage III in which these spikes of basement membrane matrix surround the subepithelial deposits, with frequent incorporation of the deposits into the capillary wall, leading to a thickened basement membrane. Stage IV is characterized by variation in electron density of the deposits and severe thickening and deformation of the glomerular basement membrane. Because of the aforementioned proteotypic digestion and degradation of the immune deposits over time, the deposits in stage IV may be less well recognized by the antisera used for immunofluorescence and the resultant staining patterns less prominent than for the other stages. Extensive effacement of foot processes, especially where they overly immune deposits, is typically present in all stages of this disorder. It should not be assumed that all cases of membranous nephropathy proceed through this sequence. For example, there may be cases of morphologic stage I that never proceed to other stages, and the occasional reports of clinical and pathologic resolution of membranous nephropathy indicate that there can be regression as well as progression, at least in the earlier stages of this process. This classification has been influential in shaping our understanding of membranous nephropathy, but it has not proven useful for guiding clinical management of patients or assessing prognosis.