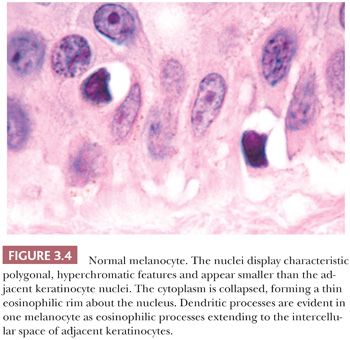
The cytologic characteristics of benign melanocytes or nevus cells are often variable. A comparison of the nuclear features of melanocytes with the features of adjacent normal keratinocyte or endothelial cell nuclei as internal references for nuclear size and detail is often helpful. The nucleus of a normal melanocyte residing within the basal layer of the epidermis typically is somewhat smaller and slightly more hyperchromatic than are the nuclei of nearby keratinocytes (Fig. 3.4) (27). The chromatin pattern is uniform, and no nucleoli are evident. The nuclear contour often appears polygonal or indented, and the cell cytoplasm appears clear as a result of artifactual retraction.
Benign melanocytic lesions do not display anaplastic cytologic characteristics. A wide spectrum of atypical nuclear changes may be seen in such lesions, but these changes generally are reactive, degenerative, or senescent phenomena rather than true anaplastic atypia characteristic of malignant transformation. True hyperchromasia, coarse nuclear membranes, and chromatin clumping are rarely encountered in benign pigment cell lesions. Certain morphologies seem to appear in most benign lesions, whereas other cytologic patterns are typical of specific lesions, such as the Spitz nevus or the pigmented spindle cell nevus.
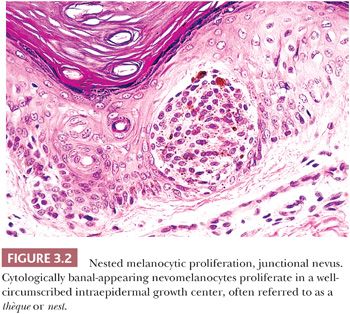
A symmetric pattern of growth and ultimately of involution is characteristic of benign melanocytic proliferations (28). Histologic symmetry from left to right is readily apparent at low power, and it includes development of the epidermal component that is congruent to that of the dermal component. Simply stated, a junctional melanocytic proliferation synchronously migrates across the dermal-epidermal junction and establishes a dermal component over a lateral dimension that is equal to that of the original epidermal component. The presence of a so-called shoulder of junctional melanocytic hyperplasia lateral to the bulk of an otherwise benign compound lesion reflects aberrant development. Such apparent histologic asymmetry may reflect the asynchronous migration of the junctional melanocyte population into the papillary dermis or the resumption of junctional melanocytic proliferation in what otherwise was a normally evolving lesion.
An apparent vertical gradient of cytologic development that is usually termed maturation is present in benign melanocytic proliferations. Cellular pleomorphism and atypical nuclear features are more evident near the epidermal origin of the tumor, with the deeper cells becoming smaller and more cytologically banal. Architectural maturation is likewise evidenced by the progressively smaller size of nevus cell nests or their replacement by infiltrating discohesive single cells at the base of the lesion.
The subsequent involution is symmetric, proceeding from top to bottom, with the replacement of dermal nevomelanocytes by normal dermal connective tissue. A wide spectrum of histologic patterns of senescence may be seen in benign melanocytic lesions. These include schwannian differentiation or neurotization, lipomatous degeneration, and inflammatory regression. A variety of cytologic patterns of senescence may also be observed, including giant cell transformation, presence of bizarre cells, and balloon cell formation. In all cases, however, such histologic patterns of senescence appear uniform and symmetric.
LENTIGO SIMPLEX
The common lentigo or lentigo simplex is a benign pigmented lesion characterized by accumulation of melanin pigment in basilar keratinocytes and melanocytic hyperplasia. Lentigines are acquired lesions, often occurring during childhood; they appear as small, uniformly dark brown macules. They may occur anywhere on the skin, but they show a predilection for acral sites.
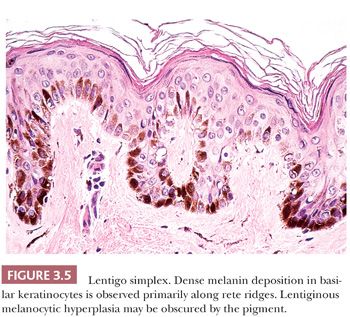
Microscopic examination reveals lentiginous melanocytic hyperplasia that is usually distributed along the tips of rete ridges (Fig. 3.5). The adjacent keratinocytes are usually hyperpigmented, and a slight degree of epidermal rete hyperplasia may be present. The histopathologic features are distinguished from those of acquired melanocytic nevi by the absence of nested melanocytes (29).
Unlike acquired melanocytic nevi and solar lentigo, lentigo simplex lesions are not associated with mutations of BRAF (V600E), PIK3CA, or FGFR3 (30).
SOLAR LENTIGO
The solar or actinic lentigo is best regarded as a pigmented lesion resulting from chronic sun exposure. There is marked variation in microscopic findings among these lesions (Fig. 3.6). Solar lentigines appear as multiple, often poorly circumscribed areas of macular hyperpigmentation that may exceed 1 cm in diameter. Histologically, besides hyperpigmentation of basilar keratinocytes, lentiginous melanocytic hyperplasia may be observed in association with marked secondary epidermal rete hyperplasia, sometimes simulating the appearance of a reticulated seborrheic keratosis. At times, however, a mixture may be seen in which epidermal atrophy alternates with areas of epidermal hyperplasia. Chronic direct ultraviolet damage may selectively affect the dispersal of melanin pigment among basilar keratinocytes.
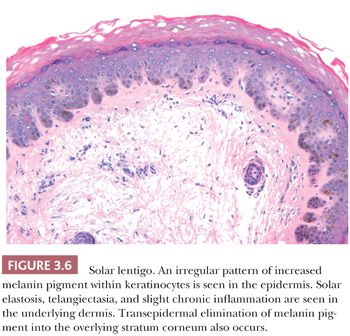
The spectrum of changes separating a benign solar lentigo from a pigmented actinic keratosis or lentigo maligna is not always clear. For this reason, caution should be exercised in reporting small biopsy specimens obtained from what otherwise appears to be a large, atypical macular pigmented lesion on chronically sun-damaged skin. The observation of cytologically benign changes within a limited tissue sample from such a lesion does not necessarily rule out the presence of adjacent histopathologic atypia.
A variant of solar lentigo is the so-called ink spot or reticulated lentigo, which can potentially be a source of confusion clinically with melanoma. Histologically, it shows lentiginous melanocytic hyperplasia and a “skipped” prominent basilar pigmentation that can focally extend to the stratum spinosus and corneum (31).
Recent studies have shown that solar lentigo and seborrheic keratosis share common mutations in FGFR3, PIK3CA, and RAS (32,33). Interestingly, these same mutations have been detected in a proportion of benign lichenoid keratosis lesions, suggesting that these lesions represent a common pattern of regression of solar lentigo and seborrheic keratosis (34).
NEVUS SPILUS
This entity, which is also known as speckled lentiginous nevus, zosteriform lentiginous nevus, or spots on a spot nevus, is usually a solitary lesion composed of a background, slightly pigmented, macule with multiple hyperpigmented papules most frequently found in the torso and extremities. It can be present at birth or develop at any age. A congenital origin has been suggested (35), but this is controversial (36). Nevus spilus tends to follow Blaschko lines (37) and can present clinically as small/medium size, giant, and zosteriform lesions (38). These lesions can be a component of neurocutaneous syndromes such as phacomatosis pigmentovascularis (var. spilorosea) (39), phacomatosis pigmentokeratotica (40), facial features, cachexia, and eye and skin lesions (FACES) syndrome (41), and the speckled lentiginous nevus syndrome (42).
Histopathologically, these lesions are composed of either lentigo simplex, melanocytic hyperplasia, or a lentiginous junctional nevic component, overlapping a background hyperpigmented macule. The speckles are represented by scattered foci of compound or dermal nevus. As can be seen in benign melanocytic processes, partial regression can sometimes be seen in these lesions.
A multitude of melanocytic lesions can be seen arising in association with nevus spilus, namely Spitz nevi (43,44), blue nevi, and even epidermal tumors such as basal cell carcinomas (45). In a recent report describing an HRAS gain-of-function mutation in a nevus spilus–associated agminated Spitz nevus, the HRAS point mutation was also detected in the background nevus spilus (44). This phenomenon of “background” HRAS mutation was also documented in nevi sebaceous, which are also known to give rise to multiple other types of epidermal tumors which show the same mutation (46). Also, one of the described neurocutaneous syndromes heavily associated with nevus spilus and nevus sebaceous, phakomatosis pigmentokeratotica, was found to be a result of mosaic postzygotic HRAS mutation (47).
Several case reports and series of nevi spili giving rise to malignant melanoma have been documented (48–52).
ACQUIRED MELANOCYTIC NEVUS
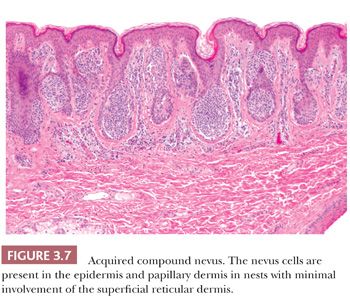
The common acquired melanocytic nevus, or mole, is the most common melanocytic tumor in humans. With increasing age, varying numbers of nevi develop in most persons (53). These usually appear as brown, pigmented lesions that are less than 0.6 cm in diameter, and they may occur anywhere on the skin surface. Common sites include the head and neck, sun-exposed trunk, and extremities. Different patterns have been described in nevi associated with specific anatomic sites. Three major histologic groupings exist that represent stages in the developmental progression of benign nevi, and these often correspond to characteristic gross morphologies. The histopathologic alterations observed in these lesions predominantly affect the epidermis and the papillary dermis.
The junctional nevus is the earliest stage of intraepidermal melanocytic proliferation. Nevomelanocytes are dispersed in multiple discrete nests along the dermal-epidermal junction, although lentiginous melanocytic hyperplasia may also be present. Junctional nevi appear clinically as small, slightly raised, and deeply pigmented lesions. The compound melanocytic nevus includes both a junctional component and the infiltration of the dermis by nevus cells distributed singly and in nests. Clinically, such lesions appear elevated or dome shaped, and they are less intensely pigmented than the junctional nevi. The dermal nevus no longer displays a junctional component; nevomelanocytes are confined to the dermis, and they are often associated with varying degrees of senescent histologic change. Clinically, they appear flesh colored or lightly pigmented, and they are dome shaped or pedunculated.
The designations junctional, dermal, and compound do not refer to discrete melanocytic entities but rather to the histologically characteristic stages in the natural progression of the common acquired melanocytic nevus. The compulsive search for a rare junctional nest of nevus cells for the sake of labeling a predominantly dermal nevus as compound nevus is wasteful, and it overlooks the significance of a developmental spectrum (54).
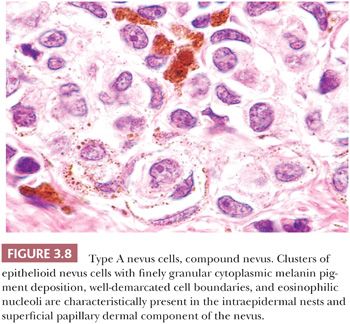
Certain cytologic features are characteristic of stages in the evolution of melanocytic nevi (Fig. 3.7). The intraepidermal nevus cell, referred to as the epithelioid melanocyte or type A nevus cell, contains a round to oval nucleus that is slightly smaller than the nuclei of the adjacent keratinocytes (Fig. 3.8). The nucleus contains finely dispersed chromatin that is similar to that of neuroendocrine cells and occasionally a single, inconspicuous nucleolus. The cytoplasm is prominent, and it often contains moderately coarse melanin granules.
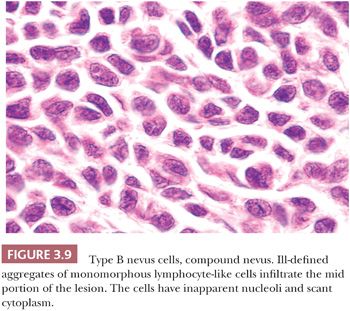
The lymphocyte-like or type B melanocyte is usually part of the dermal component of compound nevi (Fig. 3.9). The nucleus is small and round, and it contains uniformly dispersed chromatin with no apparent nucleoli. Scant, nonpigmented cytoplasm is evident.
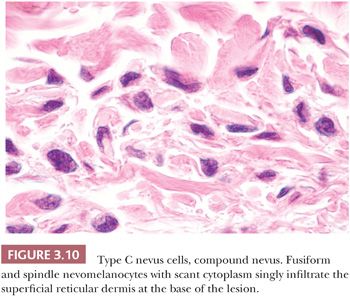
The neural or type C nevus cell is often present at the base of melanocytic lesions (Fig. 3.10). This cell is often spindle shaped, and it contains a somewhat smaller oval nucleus with a banal chromatin pattern. These fusiform cells come to rest at, or singly infiltrate, the superficial reticular dermal collagen bundles.
Different patterns of epidermal hyperplasia may be seen in association with melanocytic nevi (55,56). Acanthotic epidermal proliferation with pseudo-horn cyst formation may clinically and histologically mimic a seborrheic keratosis. Extensive reticulated epidermal retiform hyperplasia is common in some pedunculated dermal nevi.
A host inflammatory cell response may represent different phenomena. External trauma to a nevus may result from excoriation or plucking hairs. The presence of focal epidermal necrosis, impetiginization, or a foreign body response to keratinous debris suggests such a cause.
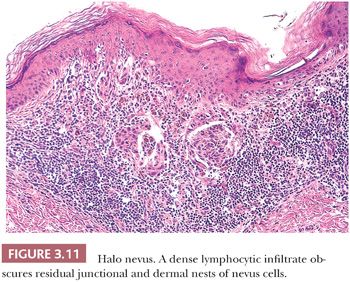
Diffuse infiltration of a nevus by lymphocytes and histiocytes with subsequent destruction of pigment-containing cells occurs in the halo nevus (57,58) (Fig. 3.11), a clinical variant in which an enlarging peripheral rim of hypopigmentation surrounds an acquired nevus (59,60). This uncommon form of symmetric inflammatory regression may be genetically determined. It should not be confused with the inflammatory host response seen in dysplastic or malignant melanocytic proliferations (see the following texts).
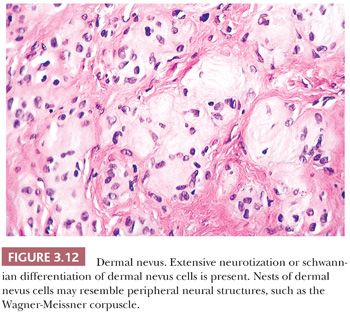
Long-term clinical observation of benign nevi reveals that most lesions undergo gradual involution (61,62). Residual dermal nevus cells are replaced by fibrous stroma. The subsequent fibrous papule or fibroepithelial polyp may contain no histologic evidence of a preexisting pigmented lesion. Melanocytic nevi on occasion display histologic and cytologic variations that reflect senescence. Prominent among the histologic senescent patterns is neurotization, or schwannian differentiation, in which the formation of neuroid structures in loose connective tissue often simulates a neurofibroma (Fig. 3.12). Other less common patterns include lipomatous degeneration, with infiltration of dermal nevi by fat cells, and osseous metaplasia. Cytologic variants including nevus giant cells or balloon cells can also be seen (63). Such variant histologic and cytologic patterns may also occur in melanoma; therefore, they should never be used as the sole point for discriminating between benign and malignant melanocytic tumors.
Most common acquired melanocytic nevi and lentigines display symmetry, uniform pigment distribution, and smooth boundaries with adjacent skin. The visual observation of asymmetry, uneven pigment distribution, and irregular or jagged borders within a pigmented lesion is usually a cause for clinical concern. Biopsy or excision is typically performed to rule out the diagnosis of malignant melanoma or a melanoma precursor. In many cases, an atypical-appearing pigmented lesion proves to be microscopically benign.
Histologically benign pigmented lesions may display subtle variations in pigment and melanocyte distribution. These may reflect physiologic or inflammatory characteristics unique to a particular lesion. The observation of such variations is significant if an otherwise histologically benign nevus or lentigo has been removed because of its worrisome clinical appearance. The diagnostic description of such features may provide valuable feedback and reassurance to the clinician.
The most common clinical cause for concern about a pigmented lesion is a reported “changing mole.” In an otherwise previously uniform symmetric nevus, such changes typically include an increase in size, the formation of irregular borders with adjacent normal skin, and a peripheral change in color. Although such clinical changes are characteristic of dysplastic nevi, a substantial percentage displays entirely benign cytologic features. Microscopically, the principal correlate is a so-called shoulder area of lentiginous junctional melanocytic proliferation beyond the lateral border of the underlying dermal component. Careful cytologic assessment of the peripheral lentiginous junctional component in addition to that of the more central domain is essential. The absence of significant anaplastic nuclear changes must be confirmed.
The biologic significance of peripheral changes within previously stable nevi is unclear. The idea that most common acquired nevi begin as junctional nevi is widely accepted. Subsequent dermal migration results in the establishment of a dermal component. The lentiginous junctional pattern of melanocytic hyperplasia is typically associated with an active, growing phase. One possibility is that, in many benign changing moles, radial junctional proliferation is reactivated. Because the clinical appearance of reactivated radial proliferation in a benign nevus may be indistinguishable from that of many dysplastic nevi and some superficial spreading melanomas, complete excision and careful histologic study are essential.
CONGENITAL MELANOCYTIC NEVUS
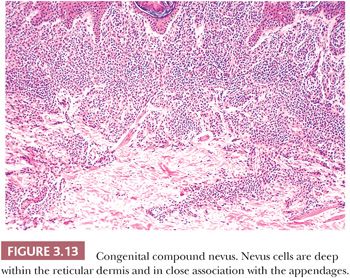
Pigmented lesions occur in approximately 1% of neonates (64). Some of these lesions are lentigines, and others are nevi. Most of the nevi are similar in size to common acquired melanocytic nevi. However, there are distinctive lesions that are quite large. Congenital melanocytic nevi are also more irregular in contour than are acquired nevi, and they are often hair bearing. These have been classified, according to size, as giant, intermediate, and small. The giant nevi can cover large areas of the body and are designated as “bathing trunk nevi” or “garment nevi.” These lesions have a somewhat increased risk for malignancy, estimated at between 5% and 10%, depending on the series (65). Histologically, compound and dermal patterns are seen. Some congenital nevi are microscopically indistinguishable from benign acquired nevi. In the most histologically characteristic lesions, the distribution of nevus cells throughout the dermis is more extensive than that seen in acquired nevi, typically involving the lower two-thirds of the reticular dermis and often infiltrating the subcutaneous fat (Fig. 3.13). The presence of nevus cells within cutaneous structures, including sebaceous lobules, multiple arrector pili muscles, and the perineurium of peripheral nerves, is characteristic of congenital nevi and is not seen in acquired nevi (Figs. 3.14 and 3.15) (66,67). The cytologic features of congenital melanocytic nevus cells and their patterns of maturation and senescence differ little from those previously described for acquired nevi (68,69).
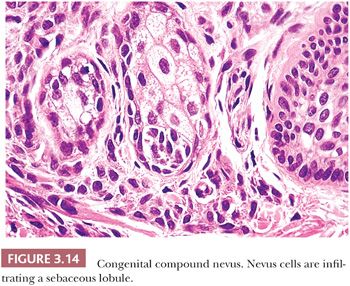
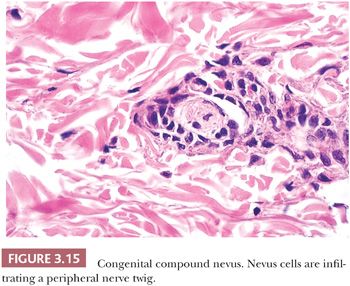
Congenital melanocytic nevi often display an irregular surface contour and pigmentation. This irregularity may reflect the distribution and density of nevomelanocytes within the underlying appendageal epithelium and arrector pili muscles (Figs. 3.13 to 3.15). Congenital nevi may appear as a localized collection of individual pigmented lesions in a nonrandom, usually linear array. Microscopically, the individual pigmented lesions comprising the array appear indistinguishable from other congenital nevi. The unusual clinical distribution most likely reflects embryologic developmental changes occurring along the Blaschko lines (70). Similar developmental changes are thought to underlie other cutaneous disorders characterized by linear and patterned arrays of discrete lesions, including inflammatory linear verrucous epidermal nevi and incontinentia pigmenti.
In most cases, the history of a pigmented lesion present from birth or the existence of easily recognizable clinical features readily permits a clinical diagnosis. Most congenital nevi are not sampled because they display asymmetric features but because they have changed in appearance. As with most acquired melanocytic nevi in which a change in size or contour is reported, the most common benign microscopic correlate observed is peripheral junctional lentiginous melanocytic hyperplasia. Another common abnormality that would prompt biopsy of a congenital nevus is the emergence of the so-called proliferative nodule. This is an expansile proliferation of epithelioid cells arising within a congenital nevus and morphologically can show benign or atypical features. Recent studies show that immunohistochemically, these lesions may represent a borderline category between nevus and melanoma (71), whereas genetic analysis shows that they have a different aberration pattern than melanomas (72). Most small congenital nevi will show a BRAF mutation; however, larger congenital nevi are more likely to show NRAS mutation (73).
Atypical pigmented lesions that are reported to be of recent or acquired onset in the nonpediatric population sometimes display features of a benign congenital melanocytic nevus. In view of the stages by which embryonic premelanocytes develop from neural crest cells and migrate to cutaneous epithelium, the microscopic observation of “congenital” features in what is clinically reported to be an “acquired” nevus may reflect a so-called latent congenital nevus. In such a congenital nevus, aberrantly migrating premelanocytes may assume a microanatomic dermal distribution characteristic of congenital nevi, without subsequent gestational proliferation. Only later in life, perhaps secondary to hormonal or developmental signals, may the melanocytic proliferation take place, yielding a clinically acquired but histologically congenital nevus.
BALLOON CELL NEVUS
This peculiar change can be found in 1.7% of all melanocytic proliferations (74,75) and can be seen in almost any type of melanocytic proliferation including intranodal nevic nests (76). This change can even be identified clinically via dermoscopy (77).
Microscopically, balloon cell change consists of a partial or complete component of cells exhibiting abundant well-demarcated, clear to foamy cytoplasm containing a small benign-appearing hyperchromatic nucleus. Melanocytic pigment can either be absent or abundant. If nuclear atypia is prominent and other features of malignancy are identified, one should suspect the rare balloon cell variant of malignant melanoma (78).
Early studies (79,80) have showed that balloon cell change resulted from melanin synthesis arrest with melanosome vacuolization due to ballooning degenerative changes probably linked with apoptotic activity (81).
ACRAL MELANOCYTIC NEVUS
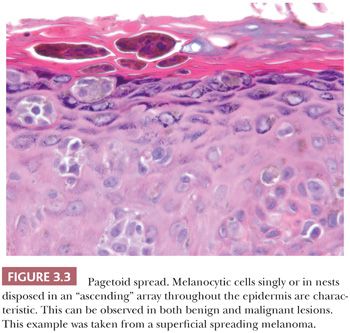
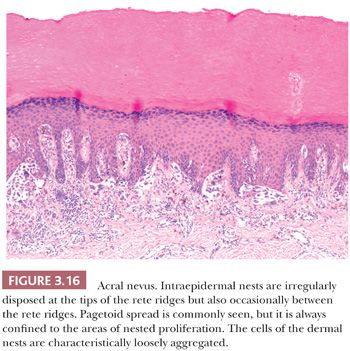
Melanocytic proliferations occurring in acral skin (hands and feet) consistently exhibit distinct characteristics that can prompt erroneous diagnosis. Dysplastic nevi and Spitz nevi rarely appear in these anatomic sites. Histologically, acral nevi present as lentiginous proliferations. The junctional nested pattern predominates. The melanocytic nests are discohesive, often irregular in size and shape, sometimes adopt a crescentic shape, and may coalesce (Fig. 3.16). Pagetoid spread is present in up to 61% of all nevi in the palm and soles (22). In the benign lesions, the areas of pagetoid ascent are restricted to the areas of nested proliferation and have benign morphology. In contrast, the pagetoid cells in acral lentiginous melanoma extend well beyond the nests in haphazard array (82–84).
NEVI OF SPECIAL SITES
A percentage of nevi arising in particular anatomic sites have been recently recognized to show unique characteristics. These nevi must be separated from compound congenital nevi, Spitz nevi, and dysplastic nevi arising at these sites. This particular group of lesions exhibits distinct and, at times, worrisome cytologic and architectural features that separate them from other nevi and are the source of possible diagnostic pitfalls. The regions that are known to produce these misleading proliferations are the head and neck, especially the periauricular area; the milk line, extending from the axilla to the groin; and the genital region, especially the vulva. The possible causes that determine these peculiar cytologic and architectural features are unknown. Speculation regarding cause has invoked developmental factors or hormonal changes such as occur in adolescence and pregnancy.
In the head and neck area, these lesions often present diagnostic challenges, especially in the scalp and ear of children and adolescents. These nevi with uncommon characteristics accounted for 10% of scalp nevi in one series (85), whereas in another series of ear and periauricular lesions, they represented 42% of the nevi occurring at this site (86). The lesions showed some common features such as poor circumscription with lateral extension of the junctional component and large irregular nests on the tips, on the sides, and in between the rete ridges. However, ear lesions tend to be symmetric and show stromal fibroplasia, whereas scalp proliferations frequently show a prominent lentiginous component with involvement of follicles and occasional pagetoid spread (87,88). In our experience, scalp lesions have intraepidermal epithelioid cell nests that are finely pigmented and have similar cells in the papillary dermis that are often mistaken for microinvasive melanoma. However, both intraepidermal and dermal cells have atypical but not malignant cytomorphology (87).
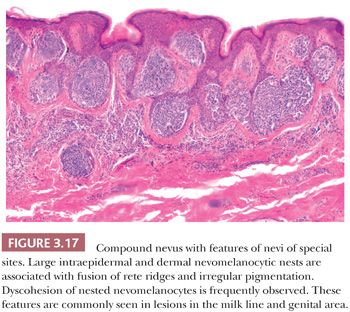
Nevi of the milk line, genital nevi, and nevi of flexural sites share several cytologic and architectural features. Nevi of the milk line comprise the axillary folds, the mammary area, the umbilicus, and the inguinal folds. Milk line and genital nevi (Fig. 3.17) share the “nested and discohesive” pattern, characterized by the presence of large, confluent nests at the dermal-epidermal junction, along with diminished melanocytic cohesion (88). Vulvar nests are often oval and lie along the rete ridges. Other distinctive changes of this group of lesions are stromal fibroplasia, which is usually coarser than in dysplastic nevi, and frequent lentiginous proliferation of the nevomelanocytes. Genital lesions, especially those occurring in the vulva, often show more prominent atypical features, such as moderate to severe cytologic atypia (epithelioid cells with prominent nucleoli) and occasional pagetoid spread. Also, the nests in these genital lesions are large, irregular, and frequently exhibit prominent pigmentation (89).
It is important to keep in mind that all these lesions show invariably benign features such as maturation and absence of dermal mitoses. These represent helpful clues to differentiate these sometimes worrisome looking but benign lesions from dysplastic nevi and malignant melanoma.
COMBINED NEVUS
On occasion, elements of two different types of dermal or epidermal melanocytic proliferation may be present in the same pigmented lesion (90). These so-called combined nevi most commonly include components of an acquired melanocytic nevus and the common blue nevus (91). Rarely, Spitz nevus cells may also be present. Combined nevi may display an aberrant histologic pattern of maturation, with pigmented epithelioid type A nevus cells at the base of the lesion instead of at the dermal-epidermal junction. Such variants should be considered in cases that otherwise appear cytologically and architecturally benign. “Inverted” type A cells should be recognized as such, and they should not be mistaken for malignant degeneration simply because they are pigmented and ectopic.
DEEP PENETRATING NEVUS
Clinically, deep penetrating nevi can occur de novo as blue papules or nodules, and often they are mistaken for a blue nevus or nodular melanoma. The more common presentation is in a preexisting acquired or congenital nevus in which a focal bluish discoloration raises the possibility of malignant degeneration. This lesion can be mistaken for malignant melanoma reportedly in as many as 40% of cases (92).
Histologically, this relatively uncommon lesion consists of a symmetric, wedge-shaped proliferation with the base parallel to the long axis of the epidermis and the vertex present in the deep dermis and sometimes in the subcutaneous fat. When it occurs de novo, there is usually an intraepidermal junctional component. When it occurs in a setting of a preexisting nevus, it usually occurs as a focal transformation in the dermal component of the preexisting nevus. The types of cells that comprise the deep penetrating nevus are more commonly the inverted type A nevus cells but sometimes are also spindle cells, type B nevus cells, and blue nevus cells. The inverted type A cells appear pale, epithelioid, and fusiform, occurring in nests often surrounded by melanophages. There is variable but usually slight melanization of the cytoplasm of the nevus cells. The cellular density in the upper dermis rapidly becomes diminished as the cells follow along the follicles or neurovascular bundles into the deeper tissue. Senescent changes often affect the inverted type A cells with nuclear vacuoles and sometimes hyperchromatic nuclei. Mitoses are very rare and, when present, should lead to thorough sectioning to rule out malignant transformation (93).
Efforts to distinguish this nevus from melanoma by molecular means have resulted in the finding of complete loss of dipeptidyl peptidase IV in malignant melanoma, compared with variable but consistent positivity in the deep penetrating nevi. However, there is a higher expression of MMP-2 and integrin beta-3 in the advancing front of deep penetrating nevi compared with that of melanoma. This finding can help explain why the pattern of infiltration resembles malignant melanoma in some cases (94).
INTRANODAL NEVIC NESTS
The finding of nests of nevic cells in lymph nodes was first reported in 1931 by Stewart and Copeland (95). The incidence of such nevic nests, known as nodal nevi, in lymph nodes draining malignancies ranges from 1% to 22% (96). In the majority of cases, nodal nevi are intracapsular or intratrabecular, but cases of intraparenchymal nests have been reported (97). The incidence of nodal nevi in sentinel lymph nodes of patients with cutaneous melanomas is 3.9%, which is higher when compared with the incidence in nonsentinel lymph nodes in melanoma patients (1.01%) (98). The origin of these nevus cells is unclear; a possible “benign metastasis-like” mechanism could be involved. One study reported a higher occurrence of nodal nevi with melanomas arising within congenital nevi (99), supporting the “benign metastasis” theory. However, more studies are needed to validate this concept. It is important to emphasize that the pathologist should be aware of the relatively high incidence of nodal nevi among sentinel lymph nodes in malignant melanoma to avoid erroneous interpretations. Besides the intracapsular and intraseptal location of the nests, perhaps the more important features that will help the pathologist make the right call is the benign cytologic and nuclear features of the nevic cells when compared with the epithelioid melanoma cells with nuclear hyperchromasia and pleomorphism.
RECURRENT MELANOCYTIC NEVUS
For cosmetic reasons, many clinicians prefer to remove acquired melanocytic nevi by a superficial “shave” biopsy and excision technique. Incomplete removal of the nevus may result in the recurrence of a pigmented lesion at the site of prior surgical trauma. Usually, such a recurrence is seen within several months of the procedure (100–105).
Recurrent melanocytic nevi often appear clinically worrisome because of the association of irregular pigmentation with scarring. When the nevus is re-excised, the specimen typically displays a residual dermal nevus component that is separated from the overlying epidermis by a zone of linear cicatricial fibrosis (Figs. 3.18 and 3.19). The overlying epidermis may display lentiginous melanocytic hyperplasia and basilar keratinocytic hyperpigmentation.
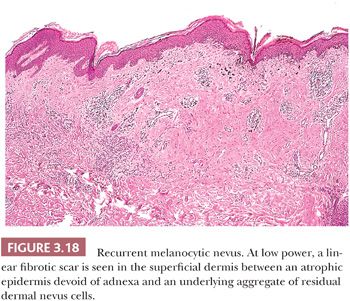
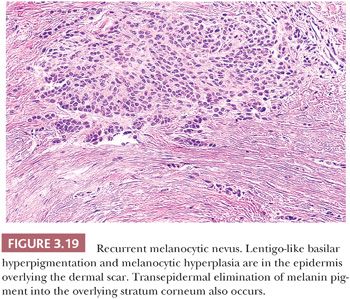
REGRESSING MELANOCYTIC NEVUS
The presence of a dense, superficial lymphoid cell infiltrate in an otherwise cytologically benign melanocytic nevus usually reflects inflammatory regression. The best-known example of such regression is the halo nevus, in which inflammatory regression occurs in a symmetric centripetal fashion (Fig. 3.11). Although the clinical appearance of a halo nevus is quite distinctive, the microscopic features may be no different from those observed in a changing pigmented lesion in which the progressive change in pigmentation is irregular or asymmetric (106).
SPITZ NEVUS AND VARIANTS
SPINDLE AND EPITHELIOID CELL NEVUS (SPITZ NEVUS)
The spindle and epithelioid cell nevus is important because of its histologic similarity to malignant melanoma. This entity was formerly known by the confusing name of benign juvenile melanoma, which nevertheless summarized its clinically benign yet histologically menacing status (107,108). This benign melanocytic lesion often referred to, at present, as the Spitz nevus may occur at all ages, although it is usually observed in children and young adults. Common sites include the head and neck and upper extremities. Clinically, this nevus presents as a small, solitary, dome-shaped dermal nodule. Because of a prominent vascular component in the tumor stroma and a relative lack of melanin pigmentation, it is frequently misdiagnosed clinically as a hemangioma or pyogenic granuloma.
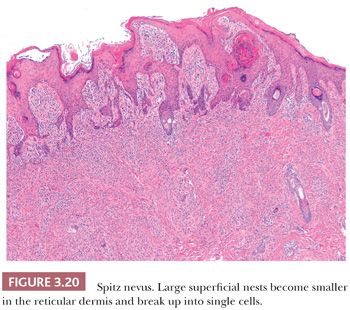
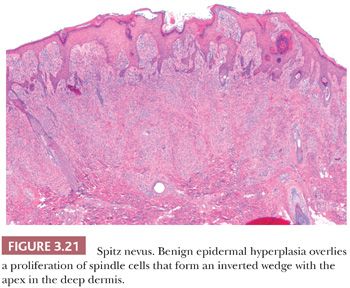
The histologic distribution of nevus cells in the Spitz nevus mirrors that of common acquired nevi, displaying junctional, compound, and dermal forms (Fig. 3.20) (109,110). However, Spitz nevi, like many congenital nevi, usually have a prominent dermal component. The overall architecture of the lesion is symmetric, with abrupt attenuation of the junctional nests at the lateral borders. The nevus is composed of varying proportions of spindle and epithelioid melanocytes. Spindle cells are usually present in fascicles arrayed perpendicular to the epidermis, whereas epithelioid cells are dispersed individually throughout the lesion. The overall histologic pattern is often discohesive and infiltrative, in contrast to the confluent and expansile growth pattern of malignant melanoma. The cells taper from a broad base superficially to a narrow “point” in the deep dermis, architecturally resembling an inverted imperfect triangle. Also, the nevus cells mature by becoming smaller from the superficial to the deep part of the tumor (Figs. 3.20 and 3.21).
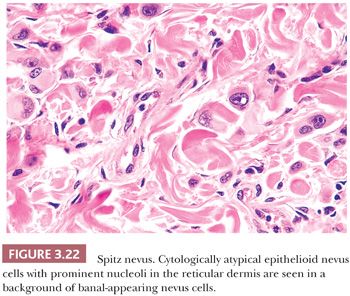
Because of its atypical cytologic features, the spindle and epithelioid cell nevus is often confused histopathologically with melanoma (111–113). An extreme degree of cellular pleomorphism, particularly of epithelioid melanocytes, may be seen (Fig. 3.22). The nuclei of these cells may be quite large and irregular in contour, and they may contain prominent eosinophilic nucleoli; however, they are otherwise open or vesicular in appearance, and they lack the coarse anaplastic features typical of malignant cells. Often present are eosinophilic cytoplasmic invaginations or “nuclear pseudoinclusions.” The presence of mitotic figures and pagetoid epidermal spread of epithelioid melanocytes may also mimic melanoma. The overwhelming majority of the cells, however, have a benign cytologic appearance.
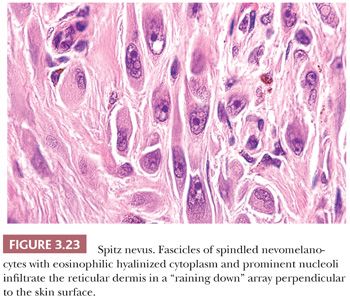
Those nevi that contain predominantly spindled melanocytes arranged in fascicles are usually more readily diagnosed than are those lesions consisting largely of pleomorphic epithelioid cells (Fig. 3.23). Several rather unique histologic features are regularly seen in Spitz nevi. These include the deposition of eosinophilic globules of hyaline-like material near the dermal-epidermal junction and the artifactual separation of papillary dermal nests from the overlying epidermis (114,115). The tumor stroma may appear quite vascular and edematous. Lymphocytic infiltration is not uncommon, and long-standing lesions may display a densely sclerotic stroma with scattered individual tumor cells trapped amid dermal collagen (116).
Histologic differentiation from melanoma may usually be made based on the overall symmetry of the lesion and the apparent cytologic “maturation” of nevus cells at the base of the lesion. The spindle and epithelioid cell nevus may rarely be a precursor of melanoma. Careful cytologic study for truly anaplastic nuclear features, bizarre mitoses, and aberrant or asymmetric proliferation should be made in borderline cases (117). At a molecular level, most Spitz nevi are composed of diploid cells and not aneuploid cells as seen in melanoma. Furthermore, except for a gain of 11p in a subset of lesions, Spitz nevi do not show significant chromosomal aberrations as seen in melanoma (118–120).
PIGMENTED SPINDLE CELL NEVUS
Regarded as a variant of the Spitz nevus by some authors, the pigmented spindle cell nevus has quite distinctive clinical and histopathologic features that justify its recognition as a separate entity (121,122). This lesion usually occurs as a small (<1 cm), solitary, deeply pigmented yet well-circumscribed maculopapule on the extremities or trunk. Women are more frequently affected than men, and the median age at diagnosis is in the third decade. The intense pigmentation results in a frequent clinical misdiagnosis of superficial spreading melanoma. Unlike the Spitz nevus, the pigmented spindle cell nevus rarely involves the head and neck.
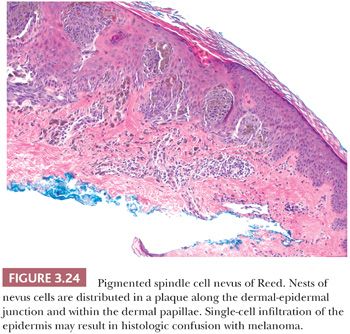
Like other benign nevomelanocytic proliferations, the pigmented spindle cell nevus displays histologic symmetry and cytologic maturation. The histologic appearance is quite characteristic (123). Nests and fascicles of spindled melanocytes arrayed parallel to the long axis of the epidermis are distributed along the dermal-epidermal junction and within the dermal papillae to form a confluent plaque (Fig. 3.24) (124). Junctional and compound varieties are exclusively seen. The base of the lesion usually extends no deeper than the superficial reticular dermis. The overall histologic pattern is one of expansile growth, as opposed to the infiltrative pattern seen at the base of Spitz nevi.
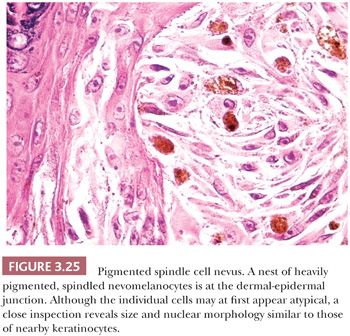
Unlike Spitz nevus cells, the nevus cells contain abundant melanin pigment, and they are often associated with dense melanophage accumulation. The nuclear characteristics are extremely monomorphous throughout the lesion. A curious cytologic trait of the pigmented spindle cell nevus is the close resemblance of the nevus cell nucleus to the nuclei of nearby epidermal keratinocytes (Fig. 3.25). The nuclei of these nevus cells are generally larger and more open in appearance than are those of the junctional cells of common acquired melanocytic nevi. One or more prominent small nucleoli are typically present. A lymphocytic response may be seen at the base of the lesion. Mitotic figures are uncommon. Although the spectrum of senescent patterns has not been fully described in these lesions, the transepidermal elimination of entire junctional nests of nevus cells may be observed (125,126).
DESMOPLASTIC (DERMAL) SPITZ NEVUS
This lesion most often appears in young adulthood, although it can be seen in any age. It usually affects the upper arms, shoulder, or the thighs but can be seen anywhere except the palms and soles. It presents as a firm nodule and is most commonly mistaken for a dermatofibroma or even a scar. It may reach 1 cm in diameter. In some cases, the lesion may even resemble a blue nevus.
Histologically, at low-power examination, one appreciates a dense collagenous stroma in which there are scattered prominent cells with large nuclei and often large nucleoli. The cells vary in shape from spindled to epithelioid. On careful inspection, the cells are found to be Spitz nevus–like cells with ample cytoplasm that are hyalinized or foamy but usually eosinophilic in appearance. The nuclei show mild hyperchromasia. Occasionally, giant cells are noted. Mitoses are rarely present. There is usually no maturation of the cells, but the cells are all contained in a striking collagenous stroma. One of the principal differential diagnostic problems is the identification of this lesion and the ruling out of desmoplastic malignant melanoma. The presence of the large epithelioid cells is most helpful in identifying the desmoplastic Spitz nevus (127).
ATYPICAL SPITZ NEVI AND SPITZ TUMORS
One of the main problems, microscopically and in the clinical setting, is the issue of atypical spindle and epithelioid cell proliferations. Clearly, some of these atypical proliferations can metastasize and relatively infrequently cause death (111,128,129). A suggested classification delineates three different groups: the atypical Spitz nevus, the atypical Spitz tumor, and the spitzoid melanomas. The first group exhibits superficial expansile nodules, lack of maturation, or a rare deep mitosis. The atypical Spitz tumor, on the other hand, is larger than a nevus, often greater than 1 cm in diameter, and extends more deeply into the dermis and subcutaneous fat. Mitoses are much more common and, in some cases, can be as frequent as 2 to 4 per mm2. For these lesions, sentinel lymph node biopsy (SLNB) is recommended. The reported range of positivity for SLNB is 26% to 50% (130–132). In our experience (unpublished data), 44% of cases were positive when an SLNB was performed. Although there is limited and short follow-up in this series, no further progression of these tumors has been reported so far (133,134).
Finally, spitzoid melanomas are predominantly dermal-based tumors with severe pleomorphism, frequent atypical mitoses, very striking expansile nodules, and often vascular invasion. These lesions are treated by excision with a 1- to 2-cm margin and an SNLB.
New information obtained from fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) analysis of atypical Spitz tumors and spitzoid melanomas are shedding new light on this very challenging area of nevomelanocytic pathology. Today, atypical spitzoid proliferations in the pediatric population can be better categorized based on cytogenetic alterations. The finding of homozygous deletion of 9p21 has been associated with aggressive behavior (135), and the finding of numerous chromosomal aberrations will favor the diagnosis of malignancy. However, a negative finding by FISH will not necessarily rule out malignancy, and to date, we still need good clinicopathologic correlation as well as long-term follow-up to completely rule out malignant behavior in the majority of borderline lesions.
DERMAL MELANOCYTOSES
Certain acquired and congenital pigmented lesions are associated with proliferative disorders of dermal melanocytes (136). Such dermal melanocytes may be derived from ectopic nests of migratory neural crest cells (137). Another possible origin postulates the possibility of nerve sheath (Schwann) cell precursors with capacity for both nerve sheath and melanocytic differentiation (138). These lesions include the common and cellular blue nevus, the Mongolian spot, and the nevi of Ota and Ito. All of these feature delicate spindle cells containing melanin granules dissecting bundles of reticular dermal collagen. The density and distribution of such dermal melanocytes, the degree of melanin pigmentation, and the presence of associated melanophage deposition usually allow the histologic discrimination of these entities (139,140). Clinically, the dermal melanocytoses display a blue or gray color that results from the absorption of the long wavelengths of visible light by dermal melanin and the reflection of the shorter blue wavelengths (Tyndall effect).
MONGOLIAN SPOT
The Mongolian spot is a congenital disorder that possibly results from the aberrant development or migration of epidermal melanocytic precursors. Clinically, the lesion appears as a diffuse area of macular blue-black uniform pigmentation over the lower back or buttocks (141). It is present at birth in most Oriental neonates, and it usually regresses within several years. Ectopic Mongolian spots may occur at other sites.
The biopsy specimens of the involved skin appear normal at low power. High-power study reveals the presence of rare elongated dermal melanocytes containing fine melanin pigment granules. The melanocytes are randomly scattered, usually in the lower two-thirds of the reticular dermis and subcutis. They are not associated with melanophage accumulation or a fibrotic reaction.
NEVUS OF OTA AND NEVUS OF ITO
These uncommon pigmented lesions are acquired hamartomas of dermal melanocytes, usually occurring in the first or second decades. They are observed most frequently in Asians, but they may also occur in Hispanics, blacks, and Native Americans. They appear clinically as macular areas of irregular blue-gray pigmentation (142). The nevus of Ota occurs in periorbital and temporal skin in the distribution of the first and second branches of the trigeminal nerve (143). The nevus of Ito occurs over supraclavicular and scapular skin in the distribution of the lateral supraclavicular and brachial nerves (144).
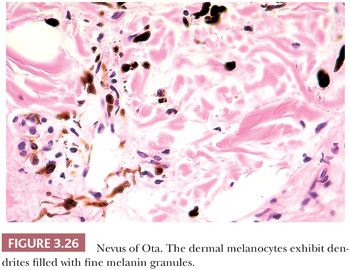
These lesions appear to be histologically distinct from the Mongolian spot. Low-power examination reveals deeply pigmented cells scattered sparsely, usually throughout the entire reticular dermis but sometimes limited only to the upper one third. Fusiform dermal melanocytes with delicate melanin pigment granules are present with the melanophages, often in a perivascular distribution (Fig. 3.26).
COMMON BLUE NEVUS
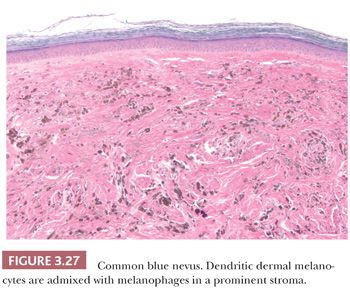
The common blue nevus is a solitary, small (<1.0 cm), slightly elevated or dome-shaped lesion that often occurs on the dorsa of the hands and feet. It occurs rarely in other organs, including the prostate and uterus (145,146). Originally described by Tièche and also known as nevus of Tièche-Jadassohn (147), this acquired lesion is usually seen in adults. A histologic examination at low power reveals a dense, well-circumscribed area of pigment deposition within the dermis (Fig. 3.27) (148). Most of the pigment is within melanophages, often obscuring the background distribution of dermal melanocytes (149). The melanocytic proliferation and pigment deposition may occupy the entire reticular dermis and occasionally the subcutaneous fat. The cells are often distributed in a periadnexal arrangement. Variable amount of sclerosis can be encountered which, if predominant, warrants the diagnosis of the sclerosing blue nevus variant (150).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


