47
CHAPTER OUTLINE
■ MEDICATIONS USED TO REDUCE OR STOP DRINKING
■ THE USE OF PHARMACOTHERAPIES IN THE TREATMENT OF ALCOHOL USE DISORDER
Over the past two decades, we have seen the entry of several medications to treat alcohol dependence. In this chapter, we review the literature on the use of medications to reduce drinking or prevent relapse in heavy drinkers. Rather than reviewing the literature exhaustively, the focus of the chapter is on developments of current interest to the clinician or that are likely to yield important clinical advances in the future. We also refer the reader to a number of other recent reviews that augment the information provided here (1–5). Of note, at the time of publication for this chapter, DSM-5 terminology had been introduced. As such, we use the DSM-5 terminology of alcohol use disorder when addressing patients who would be described as alcohol dependent by DSM-IV terminology.
The first major approach to the use of medications in the rehabilitation treatment of individuals with alcohol use disorders involves direct efforts to reduce or stop drinking behavior by producing adverse effects when alcohol is consumed or by modifying the neurotransmitter systems that mediate alcohol reinforcement. Table 47-1 lists the four medications or formulations that use this approach and are approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol use disorder. The table also shows the year of FDA approval, the presumed mechanism of action, and the approved dosage for each of these. The medications are discussed individually in the sections that follow. The second main approach to the treatment of alcohol use disorder involves the treatment of persistent psychiatric symptoms, which aims to stop or reduce drinking by modifying the motivation to use alcohol to “self-medicate” such symptoms. Medications for which this rationale underlies their use in the treatment of alcohol use disorder are discussed in the latter part of this chapter.
TABLE 47-1 MEDICATIONS APPROVED BY THE U.S. FOOD AND DRUG ADMINISTRATION FOR THE TREATMENT OF ALCOHOL DEPENDENCE
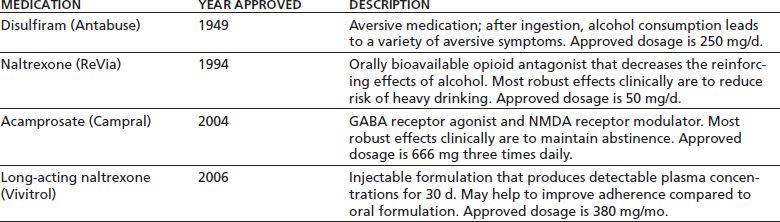
MEDICATIONS USED TO REDUCE OR STOP DRINKING
Alcohol-Sensitizing Agents
Alcohol-sensitizing agents alter the body’s response to alcohol, thereby making its ingestion unpleasant or toxic. Disulfiram (Antabuse) is the only alcohol-sensitizing medication approved in the United States for the treatment of alcohol use disorder and that is widely used clinically. Consequently, we focus on that agent here.
Disulfiram inhibits the enzyme aldehyde dehydrogenase, which catalyzes the oxidation of acetaldehyde to acetic acid. The ingestion of alcohol while this enzyme is inhibited elevates the blood acetaldehyde concentration, resulting in the disulfiram–ethanol reaction (DER). The intensity of the DER varies both with the dose of disulfiram and the volume of alcohol ingested. Symptoms and signs of the DER include warmness and flushing of the skin, especially that of the upper chest and face; increased heart rate; palpitations; and decreased blood pressure. They may also include nausea, vomiting, shortness of breath, sweating, dizziness, blurred vision, and confusion. Most DERs are self-limited, lasting about 30 minutes. Occasionally, the DER may be severe, with marked tachycardia, hypotension, or brady-cardia; rarely, it may result in cardiovascular collapse, congestive failure, and convulsions. Although severe reactions are usually associated with high doses of disulfiram (over 500 mg/d), combined with more than 2 ounces of alcohol, deaths have occurred with lower dosage and after a single drink (6–9). Concern over the potential for such effects may limit clinicians’ willingness to prescribe disulfiram.
Given its intuitive appeal, disulfiram has long been used in the rehabilitation of patients with alcohol use disorder (10), despite a lack of methodologically sound evaluations demonstrating its efficacy in the prevention of relapse. However, in selected samples of such individuals with whom special efforts, such as supervised administration, are made to ensure compliance, these medications may be useful. As discussed below, disulfiram may also limit the severity of relapse when it occurs. There are no guidelines that can be offered either to identify patients for whom disulfiram is most likely to have a beneficial effect or to match specific psychosocial interventions with particular patients to enhance compliance.
Its approval for use by the FDA preceded the implementation of rigorous requirements for efficacy that now must be satisfied for a medication to be marketed in the United States. In the controlled studies conducted, the difference in outcome between subjects receiving disulfiram and those given placebo has generally been modest.
The largest and most methodologically sound study of disulfiram was a multicenter trial conducted by the Veterans Administration Cooperative Studies Group. In that 1-year study, more than 600 male alcohol-dependent patients were randomly assigned to receive either 1 mg of disulfiram per day, 250 mg/d, or an inactive placebo (11). Patients assigned to the two disulfiram groups were told they were receiving the medication, but neither patients nor staff knew the dosage. Results showed that greater compliance with the medication regimen (in all three groups) was associated with a greater likelihood of complete abstinence. Among patients who resumed drinking, those in the group receiving 250 mg of disulfiram reported significantly fewer drinking days than did patients in either of the other two groups. Based on these findings, it appears that disulfiram may be helpful in reducing the frequency of drinking in men who cannot remain abstinent, though given the large number of statistical analyses, it is possible that this finding arose by chance (11).
Disulfiram may be of clinical value in selected individuals with alcohol use disorder with whom special efforts are made to ensure compliance. Specific behavioral efforts to enhance compliance with disulfiram (as well as other medications for the treatment of alcohol use disorder) include contracting with the patient and a significant other to work together to ensure compliance and the provision to the patient of incentives, regular reminders and other information, and behavioral training and social support (12). A trial program of stimulus control training, role playing, communication skills training, and recreational and vocational counseling improved outcome in disulfiram-treated patients compared with those receiving placebo (13). Supervision of patients being treated with disulfiram may be an essential element in ensuring compliance and enhancing the beneficial effects of the medication (14). In a 6-month study, Chick et al. (15) randomly assigned patients to receive disulfiram 200 mg/d or vitamin C 100 mg/d (ingested under the supervision of an individual chosen by the patient) as an adjunct to outpatient alcohol treatment. Treatment with disulfiram significantly increased abstinent days and decreased total drinks consumed, effects that were confirmed by parallel changes in levels of the hepatic enzyme γ-glutamyl transpeptidase.
Pharmacology and Clinical Use of Disulfiram
Disulfiram is almost completely absorbed orally. Because it binds irreversibly to aldehyde dehydrogenase, renewed enzyme activity requires the synthesis of new enzyme, so that the potential exists for a DER to occur at least 2 weeks from the last ingestion of disulfiram. Consequently, alcohol should be avoided during this period.
Disulfiram commonly produces a variety of adverse effects, including drowsiness, lethargy, and fatigue (16). Although more serious adverse effects, such as optic neuritis, peripheral neuropathy, and hepatotoxicity, occur rarely, patients treated with disulfiram should be monitored regularly for visual changes and symptoms of peripheral neuropathy and the medication discontinued if they appear. Further, the patient’s liver enzymes should be monitored monthly or at more frequent intervals during the first 3 months of treatment and quarterly thereafter to identify hepatotoxic effects, which may also warrant discontinuation of the medication. Psychiatric effects of disulfiram are uncommon and probably occur only at higher dosages of the drug, which may result in the inhibition by disulfiram of a variety of enzymes in addition to aldehyde dehydrogenase. For example, disulfiram inhibits dopamine beta-hydroxylase, which increases dopamine concentrations, which in turn can exacerbate psychotic symptoms in patients with schizophrenia and rarely result in psychotic or depressive symptoms among individuals without a psychotic disorder. Such symptoms should also lead to discontinuation of the medication.
Disulfiram is administered orally. There is a correlation between the risk of most adverse effects and dosage, although the risk of hepatic injury does not appear to be related to dose. This concern about dosage-related adverse events has resulted in the daily dosage prescribed in the United States being limited to 250 to 500 mg/d. However, efforts to titrate the dosage of disulfiram in relation to a challenge dose of ethanol have shown that some patients require in excess of 1 g/d of disulfiram to reach blood levels sufficient to produce a DER (17).
In deciding whether disulfiram should be used in treatment of alcohol use disorder, patients should be made aware of the hazards of the medication, including the need to avoid over-the-counter preparations with alcohol and drugs that can interact with disulfiram and the potential for a DER to be precipitated by alcohol used in food preparation. The administration of disulfiram to anyone who does not agree to use it, does not seek to be abstinent from alcohol, has not attained at least 48 hours of abstinence prior to first administration of disulfiram, or has any psychological or medical contraindications is not recommended. Given its potential to produce serious adverse effects when combined with alcohol, disulfiram cannot be recommended for use as part of a moderation approach to alcohol treatment.
Medications That Directly Reduce Alcohol Consumption
Several neurotransmitter systems appear to influence the reinforcing or discriminative stimulus effects of ethanol: endogenous opioids; catecholamines, especially dopamine; serotonin (5-HT); and excitatory amino acids (e.g., glutamate) (see references (1) and (4) for detailed reviews of the literature on the role of these various neurotransmitter systems in alcohol effects). Although these systems function interactively to influence drinking behavior, many of the medications that have been employed to treat alcohol use disorder affect neurotransmitter systems relatively selectively. Consequently, these systems are discussed individually here.
Opioidergic Agents
Naltrexone and, to a lesser extent, nalmefene, both of which are opioid antagonists with no intrinsic agonist properties, have been studied for the treatment of alcohol use disorder. In 1984, naltrexone was approved by the FDA for the treatment of opioid dependence; in 1994, it was approved for the treatment of alcohol use disorder. Nalmefene is approved in the United States as a parenteral formulation for the acute reversal of opioid effects (e.g., after opioid overdose or analgesia).
Naltrexone
The approval by the FDA of naltrexone for alcohol use disorder was based on the results of two single-site studies, which showed it to be efficacious in the prevention of relapse to heavy drinking (18,19). In a 12-week trial in a sample of alcohol-dependent veterans, Volpicelli et al. (18) found naltrexone to be well tolerated and to result in significantly less craving for alcohol and fewer drinking days than placebo. Among patients who drank, naltrexone also limited the progression from initial sampling of alcohol to a relapse to heavy drinking, presumably because of their experiencing less euphoric effects of alcohol, suggesting that naltrexone blocked the endogenous opioid system’s contribution to alcohol’s “priming effect” (20).
The efficacy of combining naltrexone with either supportive or cognitive–behavioral therapy (CBT) in patients with alcohol use disorder was studied by O’Malley et al. (19). This 12-week trial showed the medication to be well tolerated and to be superior to placebo in increasing the rate of abstinence and reducing the number of drinking days and relapse events and the severity of alcohol-related problems. There was an interaction effect of medication and therapy. The cumulative rate of abstinence was highest for patients treated with naltrexone and supportive therapy. However, for patients who drank, those who received naltrexone and coping skills therapy were least likely to relapse to heavy drinking.
Analysis of the potential mediating variables in these effects showed that naltrexone reduced craving for alcohol, alcohol’s reinforcing properties, the experience of intoxication, and the chances of continued drinking following a slip (21). During a 6-month, posttreatment follow-up period, the effects of naltrexone diminished gradually over time, suggesting that patients may benefit from treatment with naltrexone for longer than 12 weeks (22).
Many, but not all, subsequent studies of naltrexone have provided support for its use in alcohol treatment. The literature on naltrexone treatment of alcohol use disorder has been reviewed in detail in a number of meta-analyses (23–26). The two meta-analyses that included the largest number of studies (25,26) show a clear advantage for naltrexone over placebo on a number of drinking outcomes.
Bouza et al. (25) included 19 studies of naltrexone and a total of 3,205 participants with alcohol use disorder. The large majority of these studies were of short duration (i.e., ≤12 weeks). Using relapse as an outcome, these studies yielded a highly significant odds ratio (OR) of 0.62 (95% confidence interval [CI] 0.52 to 0.75), reflecting a 38% lower likelihood of relapse with naltrexone treatment (p < 0.00001). The likelihood of total abstinence also favored naltrexone (OR 1.26; 95% CI 0.97 to 1.64), though it did not reach statistical significance (p = 0.08). Outcomes identified as secondary by this meta-analysis, including time to relapse, percentage of drinking days, number of drinks per drinking day, days of abstinence, total alcohol consumption during treatment, and levels of gamma-glutamyl transpeptidase and aspartate aminotransferase, also showed a significant advantage for the naltrexone-treated group.
The meta-analysis by Srisurapanont and Jarusuraisin (26) included a total of 2,861 subjects from 24 randomized, controlled trials. In the short term, naltrexone significantly decreased risk of relapse to heavy drinking (relative risk [RR] 0.64, 95% CI 0.51 to 0.82) but did not reduce the likelihood of a return to any drinking (RR 0.91, 95% CI 0.81 to 1.02). Treatment with naltrexone significantly increased adverse effects, roughly doubling the likelihood of reports of nausea and dizziness and increasing the risk of fatigue by about one-third compared with placebo. However, naltrexone treatment did not significantly affect the rate of premature discontinuation of treatment (RR 0.85, 95% CI 0.70 to 1.01).
Follow-up studies of patients treated with naltrexone or placebo for 12 weeks (22,27) or 4 months (28) have shown that the medication group differences are no longer significant at posttreatment follow-up. These findings suggest that treatment with naltrexone is warranted for longer than 4 months, though the optimal duration of treatment is unknown.
An alternate approach to the use of naltrexone based on its efficacy in reducing the risk of heavy drinking among patients who continue to drink was evaluated in a study that compared the effects of naltrexone 50 mg with those of placebo in an 8-week study of problem drinkers (29). In this study, patients were randomly assigned to receive study medication either on a daily basis or for use targeted to situations identified by the patients as being high risk for heavy drinking (with the number of tablets available for use by patients in the targeted conditions decreasing over the course of the trial). Irrespective of whether they received naltrexone or placebo, patients who were trained and encouraged to use targeted treatment showed a reduced likelihood of any drinking. There was also a 19% reduction in the likelihood of heavy drinking with naltrexone treatment, suggesting that naltrexone may be useful in reducing heavy drinking, among patients who want to reduce their drinking to safe levels.
Targeted naltrexone was also used by Heinala et al. (30), who compared 50 mg/d of the medication with placebo, paired with either coping skills or supportive therapy. During an initial 12 weeks of treatment, this study showed an advantage for naltrexone in preventing relapse to heavy drinking but only when combined with coping skills therapy. During a subsequent 20-week period, subjects were told to use the medication only when they craved alcohol (i.e., targeted treatment). The beneficial effect of naltrexone on the risk of relapse was generally sustained during the period of targeted treatment. Based on these findings, it appears that targeted medication administration may be useful both for the initial treatment of problem drinking and for maintenance of the beneficial effects of an initial period of daily naltrexone.
O’Malley et al. (31) conducted a sequence of randomized trials in which subjects with alcohol use disorder were first treated with 10 weeks of open-label naltrexone 50 mg, combined with either CBT or primary care management (PCM; a less intensive, supportive approach). Treatment responders from the PCM group and from the CBT group continued in separate 24-week, placebo-controlled studies of maintenance naltrexone. No difference was observed with respect to persistent heavy drinking, with more than 80% of both groups having a positive outcome. However, the percentage of days abstinent declined more over time for the PCM group. In the follow-up studies, there was a greater maintenance response for naltrexone than placebo when combined with PCM, but the advantage for naltrexone did not reach significance when combined with CBT. These findings suggest that the beneficial effects of treatment with naltrexone can be maintained during an extended period through the use of either a more intensive, skills-oriented treatment (i.e., CBT) or a less intensive, supportive treatment combined with continued naltrexone administration.
Since naltrexone only targets certain aspects of alcohol use disorder (i.e., reduced alcohol reinforcement or cue-induced craving), there has been an interest in combining it with medications that might influence other signs/symptoms of alcoholism. Symptoms often seen after alcohol cessation are difficulty sleeping, anxiety, irritability, decreased concentration, and depressed mood. This constellation of symptoms has been called protracted withdrawal. If not addressed, the symptoms of protracted withdrawal are thought to lead to relapse to alcohol use. The anticonvulsant gabapentin may help reduce these symptoms. As such, naltrexone has been evaluated in combination with the anticonvulsant gabapentin to determine if the combination was superior to naltrexone alone and/or placebo in decreasing alcohol use.
Anton et al. (32) conducted a 16-week clinical trial of 150 alcohol-dependent subjects who were randomly assigned to naltrexone 50 mg/d alone for 16 weeks (n = 50), naltrexone 50 mg/d with gabapentin up to 1,200 mg/d for the first 6 weeks (n = 50), or double placebo (n = 50). All study patients received a combined behavioral intervention that combined cognitive–behavioral therapy, motivation enhancement, and twelve-step facilitation techniques. The results indicated that during the first 6 weeks, when gabapentin was combined with naltrexone, the combination group had a longer interval to heavy drinking than did the naltrexone alone group (which was similar to placebo), had fewer heavy drinking days than did the naltrexone alone group (which had more than did the placebo group), and had fewer drinks per drinking day than did the naltrexone alone group and the placebo group. The findings in the combination group faded over the remaining weeks of the study. There was some suggestion that the combination may work best in individuals who had previously experienced alcohol withdrawal. The investigators hypothesized that the lack of efficacy for naltrexone versus placebo may have been due to the robust psychosocial intervention (28).
Poor compliance with oral naltrexone has been shown to reduce the potential benefits of the medication (33). This has generated interest in the development and evaluation of long-acting injectable formulations of the medication. The rationale behind this approach is that monthly, compared with daily, administration would improve medication adherence and that parenteral administration would increase bio-availability by avoiding first-pass metabolism. In addition to the formulations evaluated in published studies, which are reviewed in the following sections, there are long-acting naltrexone formulations that are under development for use in the United States, Europe, and Australia.
In a pilot study, patients with alcohol use disorder treated with a subcutaneous depot formulation of naltrexone had detectable plasma concentrations of the medication for more than 30 days after the injection (34). In this study, naltrexone was superior to placebo in reducing the frequency of heavy drinking. Two long-acting naltrexone formulations administered intramuscularly have also been tested for safety and efficacy in alcohol-dependent patients. In the first study, naltrexone depot (at a dosage of 300 mg in the first month and then 150 mg monthly for 2 months) was administered in a 12-week, placebo-controlled trial in 315 patients who also received motivational enhancement therapy (35). Although naltrexone did not reduce the risk of heavy drinking, it significantly delayed the onset of any drinking, increased the total number of abstinent days, and doubled the likelihood of abstinence during the 12-week study period. Two dosage strengths of a second formulation were evaluated over 6 months of treatment in combination with a low-intensity psychosocial intervention in more than 600 individuals with alcohol use disorder who received 6 monthly injections of either long-acting naltrexone (380 mg or 190 mg) or matching volumes of placebo. Abstinence from alcohol was not required for study participation. The medication and the injections were well tolerated. Compared with placebo, treatment with the 380-mg naltrexone formulation reduced the event rate of heavy drinking by 25%, a statistically significant effect. The 17% reduction in the rate of heavy drinking produced by the 190-mg formulation did not reach statistical significance. On the basis of these findings, the FDA approved long-acting naltrexone for monthly administration at a dosage of 380 mg. Because the analysis also showed that the most robust effects of the medication were seen in patients who were abstinent (by choice) for at least a week before randomization, the package insert states that the medication should be used only in individuals with alcohol use disorder who are abstinent at treatment initiation.
A secondary analysis of data from this study examined efficacy in the subgroup of 82 patients with 4 days or more of voluntary abstinence before treatment initiation (36). This shorter period of abstinence made it possible to include a larger percentage of the study sample in the analysis than was possible initially with the use of a 7-day interval. In this study, there was a significant advantage for the 380-mg formulation compared with placebo on a number of self-report outcome measures, including greater likelihood of total abstinence (32% vs. 11%), greater median time to a first drinking day (41 days vs. 12 days), greater median time to a first heavy drinking day (>180 days vs. 20 days), lower median number of drinking days per month (0.7 vs. 7.2), and lower median heavy drinking days per month (0.2 days vs. 2.9 days). There was also a significantly greater improvement in gamma-glutamyl transpeptidase levels in the 380-mg naltrexone group. Outcomes for the 190-mg group were generally intermediate between the high-dose and placebo groups.
Clinical Considerations in the Use of Naltrexone
The clinical use of naltrexone is relatively straightforward, despite the presence of a “boxed” warning in the label concerning hepatotoxicity. The medication should be prescribed at the time that psychosocial treatment is initiated. Because of adverse effects of the medication that could compound the adverse effects of alcohol withdrawal, the initiation of naltrexone therapy is probably best delayed until after the acute withdrawal period. Initial testing for liver enzyme abnormalities is warranted to avoid prescribing the medication in the context of extreme elevations. Ongoing monitoring is required only if symptoms warrant it because the consistent effect of naltrexone in studies of alcohol use disorder has been to decrease liver enzyme concentrations.
Oral naltrexone should be administered initially at a dosage of 25 mg/d to minimize adverse effects. The dosage can then be increased in 25-mg increments every 3 to 7 days to a maximum dosage of 150 mg/d using desire to drink or another symptom that the patient identifies as reflective of risk of relapse to heavy drinking. It should be noted, however, that there is no clear evidence that a higher dosage is more efficacious than is the FDA-approved dosage of 50 mg/d. Nausea and other gastrointestinal symptoms are most common early in treatment, as are neuropsychiatric symptoms (e.g., headache, dizziness, lightheadedness, weakness), and are usually transient. Delaying or avoiding a dosage increase can be used to address more persistent adverse events. In a few patients, flulike symptoms occur, and the patient may not be willing to consider options other than discontinuation.
Long-acting naltrexone is only available as a 380-mg dose, which should be administered as a deep intramuscular injection in the upper, outer quadrant of the gluteal muscle of the buttock every 4 weeks. With repeated administrations, the injection should be alternated to the side contralateral to the immediately preceding injection. The medication is approved for use in patients who are abstinent from alcohol and who are also receiving psychosocial treatment. The precise length of the period of abstinence is not specified, and there is no evidence of any risk of consuming alcohol with naltrexone. Adverse effects with this formulation are similar to those of the oral medication, though pain and inflammation at the injection site may also occur. Local interventions, such as warm compresses, and nonsteroidal anti-inflammatory medications can be used to treat such injection site reactions.
Nalmefene
Nalmefene has also been evaluated as a treatment for alcohol use disorder. As with naltrexone, nalmefene is an opioid antagonist without agonist properties. Nalmefene’s affinity for the μ- and κ-opioid receptors is similar to that of naltrex-one, though its affinity for the δ-opioid receptor is greater than that of naltrexone (37). A pilot study of nalmefene 40 mg/d showed it to be superior to both 10 mg/d of the medication and placebo in the prevention of relapse to heavy drinking in alcohol-dependent patients (38). A subsequent study showed no difference between nalmefene 20 mg/d or 80 mg/d. However, when combined, the nalmefene-treated subjects reported significantly less heavy drinking than did the placebo group (39). A 12-week, multisite, dose-ranging study compared placebo with 5, 20, or 40 mg of nalmefene in a sample of recently abstinent outpatients with alcohol use disorder (40). In this study, all subjects showed a reduction in self-reported heavy drinking days and on biologic measures of drinking, with no difference between the active medication and placebo groups on these measures. Recently, targeted nalmefene (where subjects were encouraged to use 10 to 40 mg of the medication when they believed drinking to be imminent) was combined with a minimal psychosocial intervention in a multicenter, placebo-controlled, randomized trial (41). Nalmefene was superior to placebo in reducing heavy drinking days, very heavy drinking days, and drinks per drinking day and in increasing abstinent days. Further, after 28 weeks of treatment, when a subgroup of nalmefene-treated subjects was randomized to a withdrawal extension, patients assigned to receive placebo were more likely to return to heavier drinking.
Summary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


